The first successful enrollment in a global multicenter clinical trial of PADN treatment for chronic heart failure and pulmonary hypertension marks the pioneering launch of this technology in Europe
On June 28, 2024, local time, Pulnovo Medical launched an international multicenter clinical trial exploring Pulmonary Artery Denervation (PADN) for the treatment of chronic heart failure complicated by pulmonary hypertension. The trial successfully initiated and enrolled its first patient at Centro Hospitalar Universitário de Lisboa Central - Hospital de Santa Marta in Portugal, marking the commencement of Pulnovo Medical's innovative product deployment across multiple overseas sites!

Prof. Hang Zhang from Nanjing Medical University Affiliated Nanjing Hospital, along with Prof. Ruben Ramos and their team, successfully performed PADN procedures for 2 patients. The entire operation proceeded smoothly with straightforward instrument handling and yielded excellent surgical outcomes. The longstanding favorable performance data of PADN technology impressed the medical team in Portugal with its safety and efficacy. Following the completion of this clinical enrollment, PADN technology demonstrated favorable clinical parameters in conjunction with the new product. The comprehensive design and high-precision algorithmic control of the product greatly ensured stable energy output and surgical effectiveness during the procedures.
Case 1
Female patient, 67 years old, height 170 cm, weight 87 kg. Diagnosed with rheumatic heart disease, post-pacemaker implantation, pulmonary arterial hypertension, and post-knee joint replacement surgery. NYHA Class III, confirmed by right heart catheterization as Cpc-PH.
Pre-operative 6-minute walk distance: 138 meters. NT-proBNP: 6077 pg/ml. Echocardiogram indicated right atrial and ventricular enlargement, mild tricuspid valve stenosis, pulmonary arterial hypertension, and pericardial effusion of 9 mm. CTA revealed enlargement of the main pulmonary artery with a maximum diameter of 50 mm.
During the procedure, pulmonary artery angiography showed significant dilation of the main pulmonary artery, with a measured diameter of 47.4 mm. Based on CTA and echocardiographic findings, a 50 mm PADN catheter was selected for ablation. The surgery proceeded smoothly, and the patient reported no discomfort during the procedure. Post-ablation right heart catheterization showed a decrease in mean pulmonary artery pressure (mPAP) by 24.0% and pulmonary vascular resistance (PVR) by 42.5%. Cardiac output (CO) and cardiac index (CI) increased by 10.5%.
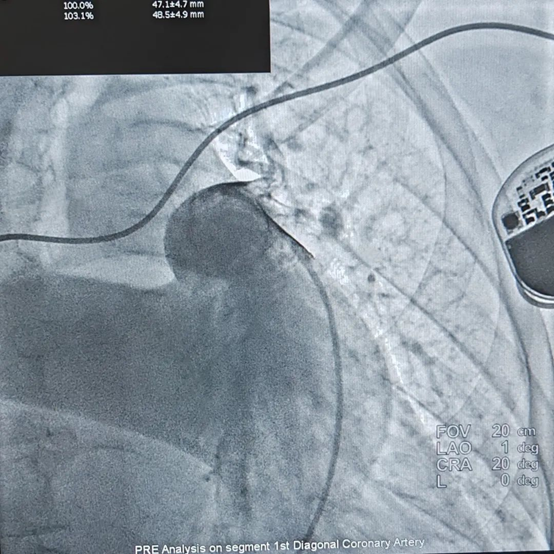
Pulmonary Artery Angiography
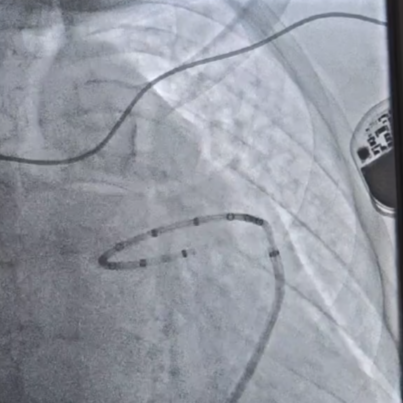
Ablation Angiography
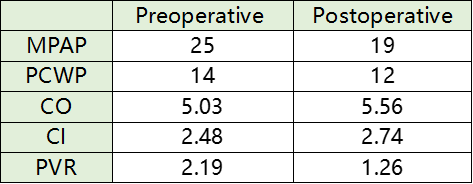
RHC hemodynamic data
Case 2
Male patient, 66 years old, height 179 cm, weight 100 kg. Diagnosed with Cpc-PH confirmed by right heart catheterization, post-coronary artery bypass grafting surgery, NYHA Class III.
Pre-operative 6-minute walk distance: 322 meters. NT-proBNP: 1333 pg/ml. Echocardiogram indicated left ventricular enlargement and pulmonary arterial hypertension. CTA showed mild dilation of the main pulmonary artery with a diameter of approximately 25 mm.
During the procedure, pulmonary artery angiography revealed a main pulmonary artery diameter of 25.8 mm. Based on CTA and echocardiographic findings, a 30 mm PADN catheter was selected for ablation. The surgery proceeded smoothly, and the patient reported no discomfort during the procedure. Post-ablation right heart catheterization showed a decrease in mean pulmonary artery pressure (mPAP) by 29.4% and pulmonary vascular resistance (PVR) by 41.8%.
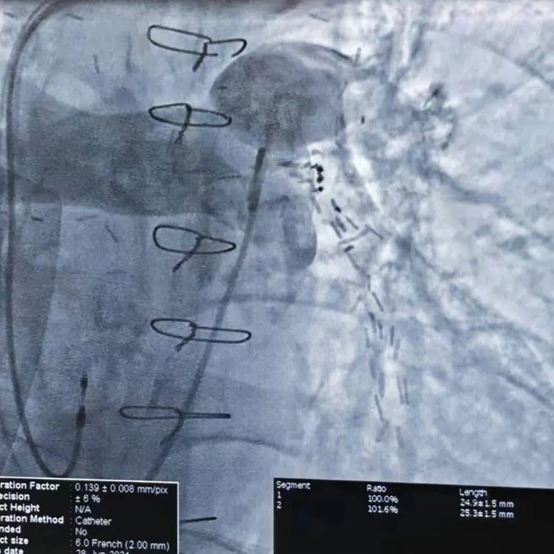
Pulmonary Artery Angiography
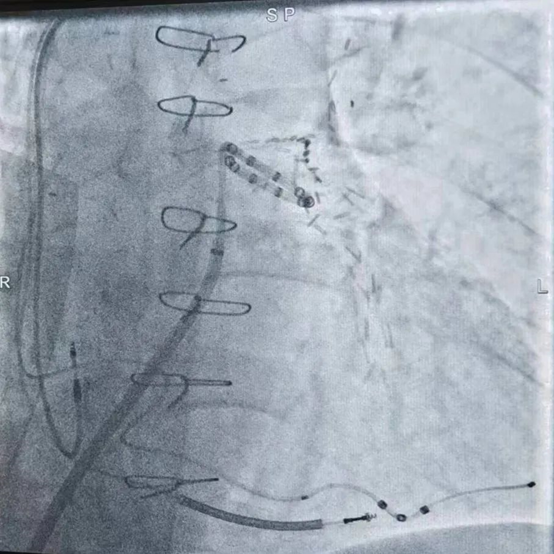
Ablation Angiography
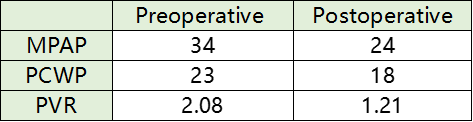
RHC hemodynamic data
The successful implementation of the first clinical enrollment not only represents an exploration in medical technology innovation but also serves to further validate the effectiveness and safety of PADN technology through this study. It aims to provide higher-quality medical services for patients and propel advancements in related disciplines.

发表留言
暂无留言
输入您的留言参与专家互动