定制的细胞外基质模拟涂层促进心脏封堵器的再内皮化和组织愈合
Yumei Qin a,1, Yun Zhu b,1, Lu Lu c, Haoshuang Wu a, Jinpeng Hu a,d, Fan Wang d, Bo Zhang a,Jian Wang e, Xia Yang e, Rifang Luo a, Juan Chen d, Qing Jiang a, Li Yang a,**, Yunbing Wang a,*,Xingdong Zhang a
a National Engineering Research Center for Biomaterials, Sichuan University, Chengdu, 610065, China
b National Key Laboratory of Biomacromolecules, CAS Center for Excellence in Biomacromolecules, Institute of Biophysics, Chinese Academy of Sciences, Beijing,100101, China
c Key Laboratory of Medical Molecular Virology (MOE/NHC/CAMS), School of Basic Medical Sciences and Shanghai Public Health Clinical Center, Fudan-Jinbo Joint Research Center, Fudan University, Shanghai, 200302, China
d Shanghai Shape Memory Alloy Co., Ltd, Shanghai, 200940, China
e Shanxi Provincial Key Laboratory for Functional Proteins, Shanxi Jinbo Bio-Pharmaceutical Co., Ltd, Taiyuan, 030032, China
ABSTRACT
Minimally invasive transcatheter interventional therapy utilizing cardiac occluders represents the primary approach for addressing congenital heart defects and left atrial appendage (LAA) thrombosis. However, incomplete endothelialization and delayed tissue healing after occluder implantation collectively compromise clinical efficacy. In this study, we have customized a recombinant humanized collagen type I (rhCol I) and developed an rhCol I-based extracellular matrix (ECM)-mimetic coating. The innovative coating integrates metalphenolic networks with anticoagulation and anti-inflammatory functions as a weak cross-linker, combining them with specifically engineered rhCol I that exhibits high cell adhesion activity and elicits a low inflammatory response. The amalgamation, driven by multiple forces, effectively serves to functionalize implantable materials,thereby responding positively to the microenvironment following occluder implantation. Experimental findings substantiate the coating’s ability to sustain a prolonged anticoagulant effect, enhance the functionality of endothelial cells and cardiomyocyte, and modulate inflammatory responses by polarizing inflammatory cells into an anti-inflammatory phenotype. Notably, occluder implantation in a canine model confirms that the coating expedites reendothelialization process and promotes tissue healing. Collectively, this tailored ECM-mimetic coating presents a promising surface modification strategy for improving the clinical efficacy of cardiacoccluders.
1. Introduction
Congenital heart defects and non-congenital left atrial appendage(LAA) thrombosis caused by atrial fibrillation are associated with high morbidity and mortality [1,2]. The advent of implantable biomaterials has revolutioned the therapies of cardiopathy, making transcatheter intervention to place occluders at the heart defects or LAA the most efficacious method of treating these diseases [3–8]. However, residual shunts caused by deformation, loosening, and displacement of the implant due to incomplete endothelialization and slow tissue healing after occluder implantation are clinically severe complications [9,10]. It is pivotal to achieve rapid surface reendothelialization and internal tissue regeneration to ensure swift closure and withstand load transfer.In addition, inflammation triggered by occluder implantation may induce rhythm disturbances, such as transient arrhythmias, and tissue erosion, which can delay myocardial healing [4,11]. Furthermore,thrombus deposition on the surface of the occluder material after contact with blood will hinder the endothelialization process [12].Therefore, how to improve the performance of the occluder to ensure its effectiveness after implantation remains a substantial challenge.
Designing surface coatings to modify occluders provides an essential entry point to improve functionality after device implantation. Studies indicate that constructing specific microstructures, such as linear patterns [13,14], or introducing bioactive molecules, such as vascular endothelial growth factor (VEGF), adhesive peptide Arg-Glu-Asp-Val(REDV), nitric oxide (NO) donors, hyaluronic acid (HA), and CD31, on the surface of the device by coatings to enhance efficacy [15–20]. Ding et al. coated titanium-nitrogen nanocoating on the surface of NiTi alloy occluder, effectively promoting the migration of endothelial cells (ECs) on the material surface [21]. Pan et al. designed surface modification by gelatin-peptide conjugate to promote endogenous tissue regeneration for biodegradable cardiac occluder [10]. While these strategies effectively improve biological function to some extent, the post-implantation environment introduces complex challenges in regulating endothelialization, thrombosis, inflammation, and tissue repair. Designing functional coatings on the surface of the occluder to achieve optimal interface responses and ultimately realize ideal cardiac tissue healing remains the focal point and challenge of current research.
Extracellular matrix (ECM) plays a pivotal role in maintaining tissue structure and regulating cell growth [22]. Developing an ECM-mimetic coating on the material surface to adapt to the implantation microenvironment may provide novel insights. Collagen type I, a fundamental component of the ECM, not only maintains structural integrity and provides mechanical support to tissues, but also participates in signal transduction [23,24]. Utilizing it to construct ECM-mimetic coatings,mimicking the composition and function of the ECM, becomes an attractive prospect. However, the prevalent collagen type I of animal origin exhibits some drawbacks, including certain immunogenicity(associated with its special structure, source, treatment, and purity),poor water solubility, and a risk of viral infection, limiting its extended application in cardiovascular settings [25,26]. In contrast, recombinant humanized collagen with designated structure and function, engineered through advanced genetic engineering, synthetic biology, and fermentation engineering, can alleviate these limitations and has shown applications in addressing atherosclerosis, myocardial infarction, and valvular heart disease [27–29]. Therefore, recombinant humanized collagen type I (rhCol I) holds as a promising candidate to construct ECM-mimetic coatings for cardiac occluders.
It is worth noting that there are many considerations on how to construct an effective way to introduce rhCol I onto the surface of the device: i) the coating should have good stability; ii) the introduction method of rhCol I should not cause the loss of its activity; iii) the coating should exhibit favorable hemocompatibility. Since collagen type I itself has no anticoagulant effect, it is necessary to develop an intermediate interface layer that can confer certain anticoagulant properties on the substrate while supporting collagen modification. Caruso et al. reported a method involving rapid coordination and one-step assembly of tannic acid (TA) and ferric ions (FeIII) into a thin film on different substrates[30,31], which has been widely used in biomedicine [32–35]. In addition to various pharmacological activities such as antioxidant,anti-inflammatory, bacteriostatic properties, and prevention of cardiovascular and cerebrovascular diseases [36,37], FeIII-TA films are abundant in phenolic hydroxyl groups and benzene rings, providing versatility in loading functional molecules through various forces [38,39]. More importantly, FeIII-TA films also presented excellent anti-platelet adhesion effects [40,41], which provides an ideal choice for surface modification of blood-contacting materials.
In light of the considerations mentioned above, we have developed a customized rhCol I and formulated an ECM-mimetic coating by intergrating metal-phenolic networks with rhCol I for biodegradable polydioxanone (PDO) occluders. The tailored rhCol I, characterized by a stable triple-helical structure and featuring pro-cell adhesion fragments(e.g., Pro-Gly-Pro (PGP), Gly-Glu-Arg (GER), and Gly-Glu-Lys (GEK))
[19,42], enhances cell adhesion activity through a tandem-repeating strategy, and shows a low inflammatory response. The metal-phenolic networks (TA-Fe), formed through the rapid coordination of TA and FeIII along with polyphenol deposition, establish a hydrophilic, anticoagulant, and anti-inflammatory platform. This rhCol I is seamlessly integrated with TA-Fe through hydrogen bond and covalent condensation,resulting in a composite coating denoted as TA-Fe/rhCol I (Fig. 1). In this work, the stability, hemocompatibility, histocompatibility, and the ability to facilitate rapid reendothelialization and myocardial tissue healing of such ECM-mimetic coating were verified through in vivo and in vitro experiments, involving occluder implantation in a canine LAA model. The results unequivocally emphasize the effectiveness of this coating in improving the functionality of cardiac occluders.
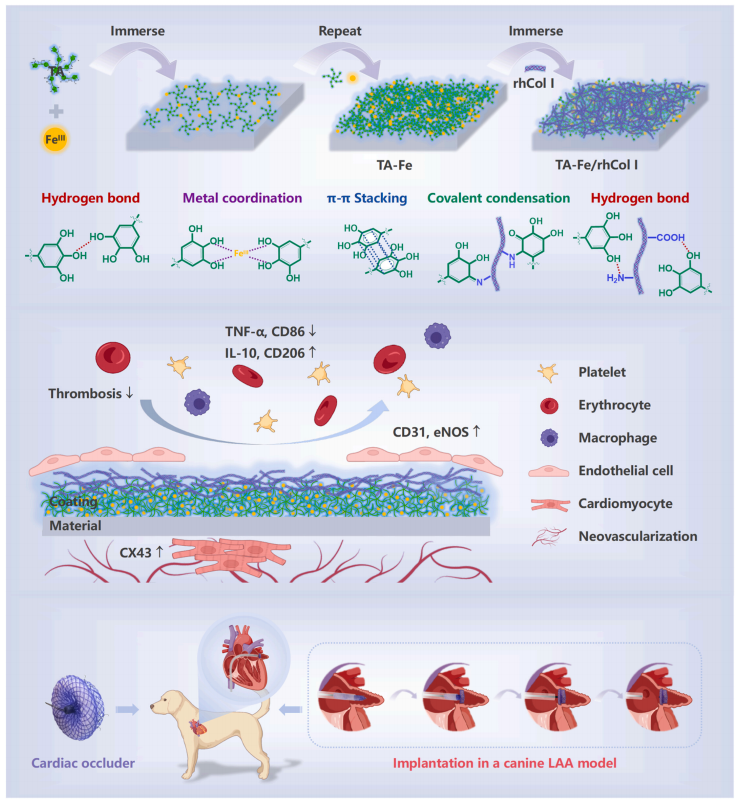
Fig. 1. Schematic illustration of the preparation process and functional model of the tailored ECM-mimetic coating of metal-phenolic networks combined with rhCol I for cardiac occluders.
2. Results and discussion
2.1. Screening and structural study of the C1P1 peptide
In addition to providing physical support, collagen also serves important physiological functions by binding various proteins, such as integrins on the cell surfaces and diverse ECM components [43]. Previous studies have identified specialized recognition motifs within triple-helical human collagen, including GFOGER, GASGER, and the "GXXGEX" motif (where X represents hydrophobic residues) [44]. Our previous research also indicated that collagen sequences containing GER or GEK motifs tend to exhibit superior structural and functional properties and are more amenable to recombinant expression [42]. In this study, we employed a similar approach and selected six candidate peptides, named C1P1–C1P6 (Table S1), from the triple-helical domain(179–1192 aa) of the human COL1A1 protein (1–1464 aa) (Fig. 2A). In addition to GER or GEK, these peptides also contain other charged residues, which are anticipated to enhance water solubility and expression efficiency of the collagen peptide. The six peptides were incubated with human umbilical vein endothelial cells (HUVECs) and rat cardiomyocyte H9C2 cells, respectively, to assess the effects of the peptides on cell growth. The cell counting kit-8 (CCK-8) results (Fig. S1)demonstrated that the adhesion and proliferation activities of HUVECs and H9C2 cells in the C1P1 group were significantly higher than those in the other peptide groups at both 1 and 3 days, indicating that the C1P1 peptide had the highest potential for promoting cell growth. Consequently, the C1P1 polypeptide was selected for subsequent investigations.
The C1P1 peptide is a fragment (G932–S991) of the human COL1A1 protein, specifically the alpha 1 chain of human collagen type I (Fig. 2A).While natural human collagen type I exists as a heterotrimeric complex,the synthesized peptide and subsequent recombinant protein used in this study are both homogeneous and can only form a homotrimer complex.Therefore, it is necessary to investigate whether the C1P1 peptide sequence derived from the human COL1A1 protein can form a regular collagen triple-helical structure. Protein crystallography is currently the primary method used to study the high-resolution structure of the collagen triple helix. To screen crystals, we divided the C1P1 polypeptide into multiple short peptides. Eventually, we successfully screened high-quality single crystals by using the C1P1S short peptide(965–974 aa) within the C1P1 sequence and determined its crystal structure (Table S2). This C1P1S peptide is highly conserved across different species (Fig. 2B and S2), suggesting its significance in the functions of human collagen type I. Interestingly, we also observed a high similarity between the C1P1S motif and its counterpart in the COL1A2 protein (Fig. 2C), which may be associated with its structural
stability or binding affinity to the target cell.
Using the host-guest peptide strategy, the crystal structure of the(POG)3-QRGERGFPG-(POG)3 peptide was solved to 1.54 Å resolution(Table S2). This structure adopts a standard triple-helical conformation,and we can identify the leading, middle, and trailing chains within the helix. In addition to the common backbone hydrogen bond used to form the triple-helical structure, we observed several strong interactions between the side chains of residues from different chains (Fig. 2D). For example, Gln966 and Glu969 of the trailing strand interact with Arg967 and Arg970 of the middle strand, Gln966 and Glu969 of the middle strand interact with Arg967 and Arg970 of the leading strand, and Glu969 and the backbone carbonyl oxygen of the leading strand interact with Arg967 and Arg970 of the trailing strand. These results showed that the C1P1S motif can form a stable triple-helical configuration through both backbone and side chain interactions. Structural alignment of the whole molecule (Fig. 2E) revealed that the C1P1S triple helix closely resembles other collagen structures, except for a greatly bent helical structure (PDB entry 6A0C) [42]. Therefore, it suggested that the homogeneous C1P1 peptide might also form a regular triple-helical structure. Then, using the tandem-repeating strategy, a tailored rhCol I containing four copies of the C1P1 sequence(GEKGSPGADGPAGAPGTPGPQGIAGQRGVVGLPGQRGERGFPGLPGPSGEPGKQGPSGAS)was constructed and purified to further enhance the structural stabilityand activity in modulating the cellular behavior. The molecular weight of rhCol I can be found in Fig. S3.
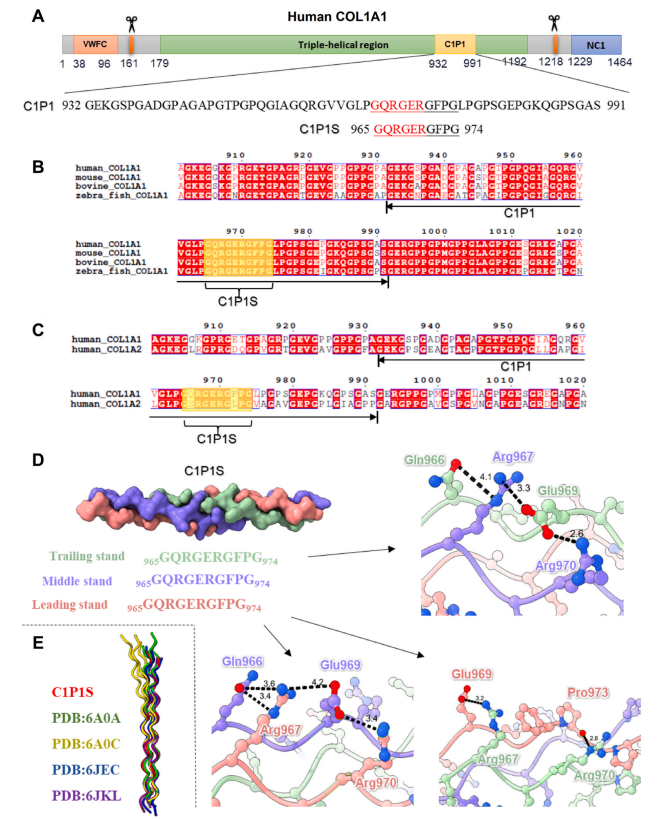
Fig. 2. Structural analysis of the C1P1S motif in the C1P1 peptide of the human COL1A1 protein. (A) Diagram illustrating the distribution of domains in the human COL1A1 protein, with the amino acid sequences of the C1P1 and C1P1S peptides displayed. (B) Sequence alignment of COL1A1 proteins from various species.(C) Sequence alignment of human COL1A1 and COL1A2 proteins. (D) The triple-helical structure of the C1P1S peptide, with interactions between residues denoted by black dashed lines and important residues labeled. (E) Structural comparisons between the C1P1S peptide and some previously reported collagen structures.
2.2. Preparation and characterization of the ECM-mimetic coating
The preparation of the ECM-mimetic coating (denoted as TA-Fe/rhCol I) involved a rapid and spontaneous two-step process (Fig. 1).The TA-Fe coating formed through the swift coordination of TA and FeIII,which completed almost instantaneously, with bis-complex dominating at pH 3–6 [30], along with polyphenol deposition driven by hydrogen bond, π-π stacking, and metal coordination facilitated by abundant phenolic hydroxyl groups (–OH) and benzene rings in TA. The tailored rhCol was combined with TA-Fe via numerous hydrogen bonds and covalent condensation to yield a TA-Fe/rhCol I coating.
Surface morphologies of the bare, TA-Fe, and TA-Fe/rhCol I coated PDO substrates were observed using field emission scanning electron microscopy (FE-SEM) and atomic force microscopy (AFM). As shown in Fig. 3A and C, nanoparticles appeared on the TA-Fe coating due to the coordination of TA and FeIII in solution to form metal-phenolic nuclear complexes, which continuously grew into nanoparticles and then deposited and fused onto the substrate [45]. With the introduction of rhCol, the TA-Fe/rhCol I coating presented a more uniform and dense arrangement of nanoparticles, accompanied by increased surface roughness and coating thickness (Fig. 3D and E). Energy dispersive spectroscopy (EDS) (Fig. 3B) exhibited signals of C, O, Fe, and N on the TA-Fe/rhCol I surface, indicating successful loading of FeIII and rhCol I.Water contact angle (WCA) results (Fig. 3F) revealed significantly improved hydrophilicity after TA-Fe introduction, diminishing upon rhCol I modification. Surface zeta potential (Fig. 3G) of the TA-Fecoating decreased due to electronegative polyphenols, while rhCol I partially neutralized the TA-Fe coating’s negative charge, increasing the potential of the TA-Fe/rhCol I coating.
The chemical composition of the coatings was evaluated using attenuated total reflection Fourier transform infrared (ATR-FTIR)(Fig. 3H and S4). For the TA-Fe coating, the broad absorption peak at3690–3125 cm−1signified the stretching vibration of phenolic –OH,while the peaks at 1612 cm−1 and 1536cm−1 represented the stretchingvibration peaks of the benzene ring skeleton. The broad absorption peakof the TA-Fe/rhCol I coating (3321 cm−1) exhibited a slight red-shift compared to the TA-Fe coating (3394 cm−1) due to the introduction of carboxyl groups (–COOH) and amino groups (–NH2) in the rhCol I molecules, resulting in new hydrogen bonds. Moreover, the C=O stretching vibration peak (amide I band) and N–H bending vibration peak (amide II band) of the rhCol I amide group were observed at 1640cm−1 and 1536 cm−1, respectively, in the TA-Fe/rhCol I coating. X-ray photoelectron spectroscopy (XPS) results (Fig. 3I) showed that C, O, and Fe signals were detected in both coatings, with an additional N signal appearing in the TA-Fe/rhCol I coating. Fig. S5 indicated that the ratio of N in the TA-Fe/rhCol I coating increased, whereas the ratios of O and Fe decreased due to the introduction of rhCol I covering the TA-Fe coating to a certain extent. In addition, the C1s high-resolution spectra (Fig. 3J–L and Table S3) showed a new π bond peak of the benzene ring skeleton in the TA-Fe coating. In comparison, the proportions of the π bond peak and the C–O peak of polyphenol decreased in the TA-Fe/rhCol I coating, whereas the proportions of the C=O peak and the C–N peak attributed to rhCol I increased significantly. These results confirmed the successful loading of TA, FeIII, and rhCol I, as well as the successful preparation of the ECM-mimetic coating.
The dynamic assembly process of the ECM-mimetic coating was monitored in real time using a quartz crystal microbalance with dissipation (QCM-D). As shown in Fig. 3M, the TA-Fe and rhCol I were successfully assembled on gold-plated quartz crystals with densities of 720.48 ng/cm2 and 139.52 ng/cm2, respectively, with almost no mass loss after rinsing with ultrapure water. Finally, the assembled coating exhibited stable adsorption after phosphate-buffered saline (PBS) erosion. Furthermore, fluorescein isothiocyanate (FITC)-conjugated rhCol I was incorporated into the coating to evaluate its stability (Fig. 3N and O). Uniform and dense green fluorescence was observed on the surface of freshly prepared FITC-conjugated TA-Fe/rhCol I coating. The fluorescence density was not significantly reduced after immersion in PBS for 7 days (86.40 ± 7.89 %), and a large number of fluorescence signals still remained after 14 days (70.33 ± 5.96 %), indicating that the TA-Fe/rhCol I coating had sufficient stability to function during the critical first two weeks after material implantation. In addition, the loading amount of rhCol I in the coating was determined using the bicinchonininc acid (BCA) assay [39]. Fig. 3O showed that after immersion in PBS for 0, 7, and 14 days, the densities of rhCol I in the TA-Fe/rhCol I coatings were 103.41 ± 7.28 μg/cm2, 90.76 ± 6.43μg/cm2, and 73.18 ± 7.01 μg/cm2, respectively, where over 70 % of rhCol I remained after 14 days, which was consistent with the statistical results of fluorescence images, confirming the stability of rhCol I loading. The –OH of TA can form numerous hydrogen bonds with the–COOH, –NH2, and peptide bonds (–CO–NH–) of rhCol I, and the benzoquinone groups generated by the oxidation of phenolic –OH under weak alkaline conditions can be covalently condensed with the –NH2 of rhCol I through Schiff base and Michael addition reactions [46,47],thereby realizing efficient and stable loading of rhCol I.
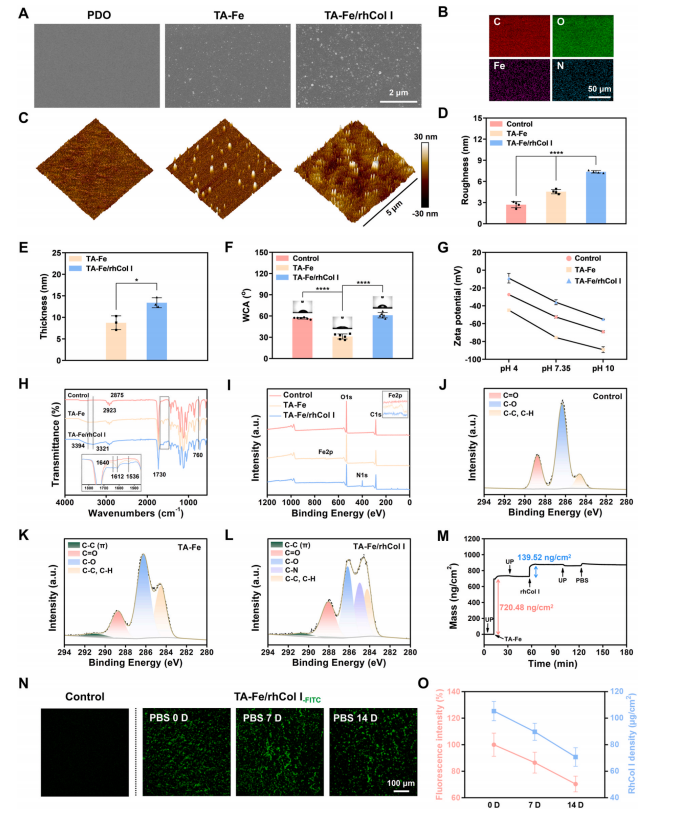
Fig. 3. Characterization of the ECM-mimetic coating. (A) SEM and (C) AFM images of the bare, TA-Fe, and TA-Fe/rhCol I coated PDO substrates. (B) Elemental mappings of the TA-Fe/rhCol I coating observed by EDS. (D) Surface roughness of different samples. (E) Thickness of the TA-Fe and TA-Fe/rhCol I coatings. (F)Surface hydrophilicity and (G) surface zeta potentials of different samples. (H) ATR-FTIR spectra, (I) XPS survey scan spectra, and (J–L) XPS high-resolution C1s spectra of the bare, TA-Fe, and TA-Fe/rhCol I coated PDO substrates. (M) Real-time mass variation of the TA-Fe/rhCol I coating on gold-plated quartz crystal monitored by QCM-D. (N) Fluorescence images of FITC-conjugated TA-Fe/rhCol I coating after immersion in PBS for 0, 7, and 14 days. (O) Quantitative statistics of fluorescence intensity and rhCol I density of the coating after immersion in PBS for 0, 7, and 14 days. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
2.3. Hemocompatibility of the ECM-mimetic coating
Anticoagulation stands as a requisite for implantable cardiovascular devices [3]. The aggregation and activation of blood cells are closely related to thrombosis [48,49]. Platelets and whole blood adhering to the sample surfaces were depicted in Fig. 4A. The bare PDO monofilaments adhered to numerous activated platelets with extended pseudopods and aggregated erythrocytes, in contrast to the TA-Fe and TA-Fe/rhCol I surfaces where only a limited number of platelets and dispersed erythrocytes adhered (Fig. 4B and C). Both platelet count (Fig. 4D) and lactate dehydrogenase (LDH) quantitative statistics (Fig. 4E) indicated significantly reduced platelet adhesion in the coating groups. Devices always encounter the risk of coagulation before endothelialization, hence the long-term anticoagulant efficacy is still worthy of attention [48]. After immersing the samples in PBS for 7, 14, and 28 days, the coating groups,especially the TA-Fe/rhCol I coating, demonstrated sustained effectiveness in preventing platelet adhesion compared to the uncoated control (Fig. S6).
P-selectin is expressed on the surface of activated platelet membrane, serving as a specific marker reflecting the degree of platelet activation [50]. The immunofluorescence analysis of P-selectin expression in platelets on the sample surface was conducted to assess platelet activation. As shown in Fig. 4F and G, and S7, platelets adhered to the bare PDO surface mostly exhibited an activated state with high expression of P-selectin, whereas the expression of P-selectin was significantly decreased in platelets adhered to the TA-Fe and TA-Fe/rhCol I surfaces, indicating lower platelet activation. With prolonged soaking time of the coatings in PBS, the expression of P-selectin and the activation degree of platelets in the coating groups increased to a certain extent, but compared to the bare PDO group, the TA-Fe/rhCol I coating still significantly inhibited platelet adhesion and activation for up to 28 days, indicating that the coating has a long-term anticoagulant effect.
The antithrombotic performance was evaluated through a ex vivo blood circulation experiment in rabbit (Fig. 4H) [27]. SEM results(Fig. 4I) illustrated a severe thrombus composed of activated platelets,erythrocytes, and fibrin on the uncoated control, whereas only sparse platelets were present on the TA-Fe and TA-Fe/rhCol I coatings. Optical photographs (Fig. 4I) and statistical analysis (Fig. 4J) revealed a substantial and visible thrombus on the control surface, with a severe occlusion of 31.98 ± 3.94 %, which was significantly mitigated in the coating groups (3.16 ± 1.73 % for the TA-Fe coating and 2.41 ± 1.68 % for the TA-Fe/rhCol I coating), indicating that the coatings could inhibit thrombosis.
Adsorption and denaturation of plasma proteins on the surface of implanted materials can trigger chain reactions, such as platelet adhesion, activation, and thrombosis [51]. To further explore the antithrombotic effect of the coatings, FITC-conjugated bovine serum albumin (BSA-FITC) and FITC-conjugated fibrinogen (FBG-FITC) were adsorbed onto the material surfaces. Fluorescence images (Fig. 4M) and intensity analysis (Fig. 4K) depicted strong fluorescence on the control surface, whereas the fluorescence intensities on the TA-Fe and TA-Fe/rhCol I surfaces were notably weakened, indicating reduced adsorption of BSA and FBG by the coatings.
Hemolysis performance is also an important index to evaluate the hemocompatibility of materials [51]. As shown in Fig. 4L, the hemolysis rates of all samples were below 1 %, meeting the safety standard of less than 5 % as specified in ISO 10993-4 [51]. Therefore, the materials and coatings have no hemolysis risk and are safe after implantation.
To sum up, these findings confirmed the effective anticoagulation performance and hemolysis safety of the ECM-mimetic coating,demonstrating its excellent hemocompatibility. The metal-phenolic networks as an intermediate interface layer, exhibit good hydrophilicity and can form a hydration layer to prevent the adsorption of plasma proteins [47,52,53]. In addition, phenolic can inhibit the conformational change of adsorbed proteins [40,41]. Both factors facilitated the resistance of the ECM-mimetic coating against subsequent adhesion and activation of platelets, thereby preventing thrombosis.
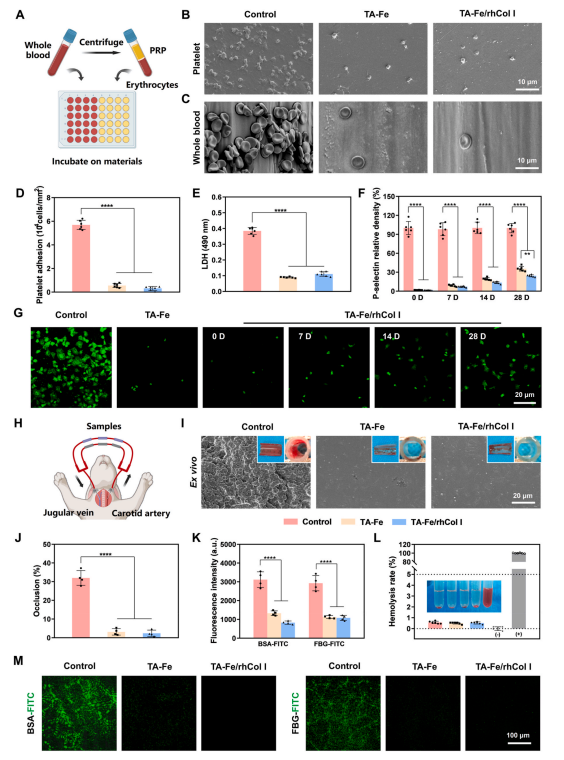
Fig. 4. Evaluation of hemocompatibility of the ECM-mimetic coating. (A) Schematic of the in vitro static platelet and whole blood adhesion tests. SEM images of (B) platelets and (C) whole blood adhering to the sample surfaces. Assessment of (D) platelet density and (E) LDH quantification on the sample surfaces.(F)Quantitative statistics and (G) immunofluorescence images of P-selectin expression in platelets adhering to different sample surfaces. (H) Schematic of a ex vivo blood circulation experiment in rabbit via an arteriovenous shunt model. (I) Photographs of sample surfaces and catheter cross-sections, along with surface SEM images of different samples after ex vivo blood circulation for 2 h. (J) Statistical analysis of catheter occlusion rates. Fluorescence (K) intensities and (M) images of BSA-FITC and FBG-FITC adsorbed on the sample surfaces. (L) Statistics of hemolysis rates for different samples.
2.4. Growth behavior of HUVECs
The endothelial damage caused during device implantation triggers the risk of thrombosis and inflammation, which is detrimental to tissue healing at the implantation site [49]. Rapid reendothelialization is crucial for ensuring the functionality of the devices [27,48]. The endothelialization capacity of the ECM-mimetic coating was assessed through in vitro evaluating the proliferation and migration of HUVECs.Fluorescein diacetate (FDA) and propidium iodide (PI) were used to stain live and dead cells, respectively, and the co-staining results (Fig. 5A) showed that HUVECs survived well on all sample surfaces without significant cell death, indicating that the materials and coatings exhibited no cytotoxicity towards HUVECs. The results of the CCK-8 assay (Fig. 5C) demonstrated a significant enhancement in HUVEC proliferation on the TA-Fe/rhCol I coating compared to the uncoated surface. Moreover, the cell migration results (Fig. 5B and D, and S8)showed that HUVECs achieved 100 % migration on the TA-Fe/rhCol I surface after 2 days under serum-free conditions, in contrast to the 63.3% migration observed in the control group. Studies have suggested that TA contains pyrogallol and catechol structures that facilitate cell adhesion [52], and TA effectively mitigates oxidative stress on material surfaces, thereby reducing oxidative damage to HUVECs [18,54]. The tailored rhCol I contains highly cell-adhesive segments, such as PGP,GER, and GEK, which can bind to integrins on the cell membrane,promoting cell adhesion. The use of a tandem-repeating strategy further enhances the adhesion activity of rhCol I to cells [19,42,44]. These results indicated that the TA-Fe/rhCol I coating promoted the adhesion,proliferation, and migration of HUVECs, demonstrating its potential to accelerate endothelialization.
In addition, a tube formation experiment using HUVECs was conducted to evaluate the effect of the ECM-mimetic coating on angiogenesis, which is vital for supplying nutrients and transporting cytokines during tissue repair and regeneration [28,55]. As shown in Fig. 5G, the TA-Fe and TA-Fe/rhCol I groups exhibited more tube formation than the control group. The number of junctions and the total tube length serve as key indicators for evaluating tube formation effects [55], and both parameters were significantly increased in the coating groups (Fig. 5E andF). It has been reported that VEGF can promote the growth of vascular endothelial cells and enhance angiogenesis [56]. The Western blot results (Fig. 5H) showed that VEGF expressions in HUVECs of the coating groups were significantly higher compared to the control group. These results suggested that the TA-Fe/rhCol I coating promoted angiogenesis by enhancing VEGF expression, contributing to the tissue healing process after occluder implantation. Studies have shown TA and Fe may up-regulate the expression of angiogenesis-related genes in HUVECs through associated signaling pathways [56–60]. In addition, angiogenesis is highly dependent on adhesion interactions of vascular endothelial cells, and rhCol I can mediate cell-cell and cell-ECM interactions via binding integrins [42,57]. These factors together contribute to the pro-angiogenic effect of the ECM-mimetic coating.
2.5. Growth behavior of H9C2 cells
The growth behavior of cardiomyocytes significantly influences the repair process of the cardiac tissue after occluder implantation [61]. The survival, adhesion, and proliferation of H9C2 cells on different sample surfaces were evaluated using FDA/PI co-staining (Fig. 5J) and the CCK-8 assay (Fig. 5M). Only very few dead cells were observed on the sample surfaces, indicating that the samples were not cytotoxic to H9C2 cells. Notably, the TA-Fe/rhCol I group exhibited superior cell viability, attributed to the synergistic effects of TA’s antioxidation capabilities[18,54] and rhCol I’s pro-cell adhesion properties [19,42,44]. Native cardiomyocytes typically display elongated shapes with organized myofibrils, reflective to their contractile nature [61]. H9C2 cytoskeleton staining (Fig. 5K) and quantitative analysis of cell area (Fig. 5N) and aspect ratio (Fig. 5O) showed that H9C2 cells in the TA-Fe/rhCol I group exhibited a more regular actin arrangement, a more expansive morphology (1507.35 ± 535.24 μm2), and a larger aspect ratio (2.69 ±0.81) compared to the control group.
Furthermore, as the principal connexin in the cardiomyocyte gap junctions and a vital electrical coupling protein in the myocardium,connexin 43 (CX43) is crucial for the rapid anisotropic conduction of action potentials required for synchronous myocardial contraction [62,63]. As shown in Fig. 5L and P, the expression level of CX43 in H9C2 cells in the TA-Fe/rhCol I group significantly surpassed that in the control and TA-Fe groups, which may be related to the function of rhCol I in promoting cell adhesion [42,44]. The Western blot results (Fig. 5I) were consistent with those of the immunofluorescence analysis. Therefore, the ECM-mimetic coating significantly improved the functionality of cardiomyocytes.
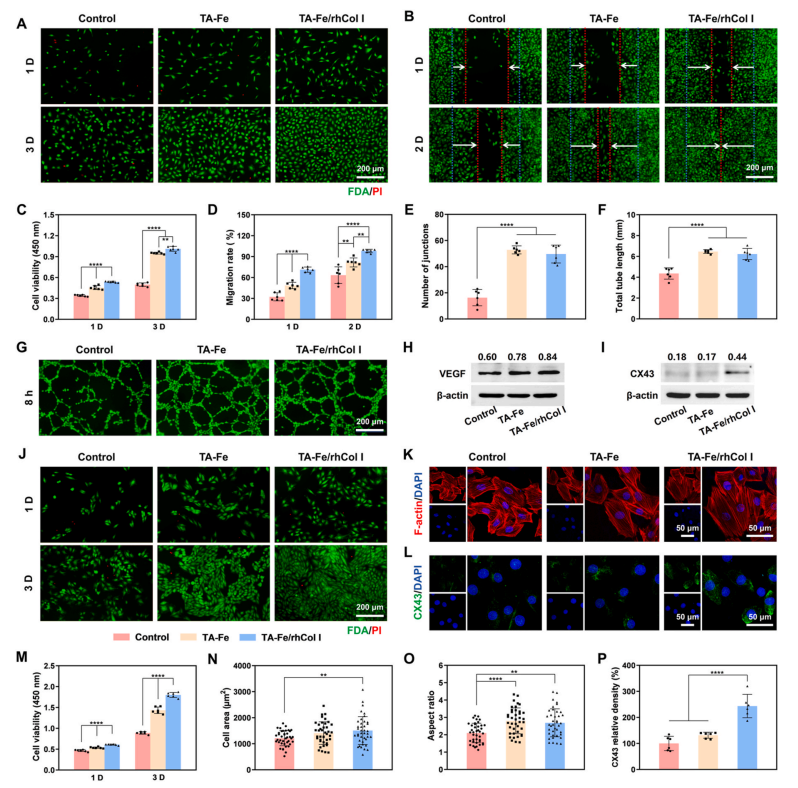
Fig. 5. Evaluation of growth behaviors of HUVECs and H9C2 cells. (A) FDA/PI co-staining images and (C) cell viabilities of HUVECs cultured on the sample surfaces after 1 and 3 days. (B) Fluorescence images and (D) migration rate statistics of HUVEC migration on the sample surfaces after 1 and 2 days. Quantitative statistics of (E) number of junctions and (F) total tube length, and (G) fluorescence images of HUVEC tube formation on Matrigel surfaces for 8 h. Western blot analysis of (H) VEGF expression in HUVECs and (I) CX43 expression in H9C2 cells (normalization with β-actin as the reference). (J) FDA/PI co-staining images and (M) cell viabilities of H9C2 cells cultured on the sample surfaces after 1 and 3 days. (K) Confocal laser scanning microscopy (CLSM) images of H9C2 cytoskeleton and quantitative statistics of (N) cell area and (O) cell aspect ratio after 3 days of culture on the sample surfaces. (L) Immunofluorescence images and (P) quantitative statistics of CX43 expression in H9C2 cells cultured on the sample surfaces after 3 days.
2.6. Inflammatory response
An excessive inflammatory response post-material implantation hinders in situ endothelialization and tissue healing [64]. A large number of macrophages accumulate and polarize at the implantation site, releasing proinflammatory factors, which may induce endothelialization disorder and excessive tissue hyperplasia; hence, the regulation of macrophages and inflammatory factors on material surfaces is pivotal [49]. The expressions of CD86 and CD206, typical markers of classically activated macrophages (M1) and alternatively activated macrophages (M2) [27], respectively, in RAW 264.7 cells were analyzed through immunofluorescence staining (Fig. 6A). CLSM images (Fig. 6B) and relative density statistics (Fig. 6E) showed that cells adhering to the control surface predominantly exhibited an activated state with extended pseudopods, expressing more CD86 and less CD206 compared to the coating groups. This suggested that the control surface was more likely to stimulate macrophage polarization towards the proinflammatory M1 phenotype. In contrast, cells in the TA-Fe and TA-Fe/rhCol I groups showed an unactivated spherical shape, suggesting a less inflammatory response on the coated surfaces.
Subcutaneous implantation serves as a common model for studying inflammatory response at the site of material implantation [27]. The inflammatory response in vivo of the ECM-mimetic coating was evaluated by implanting PDO monofilaments into the subcutaneous tissue on the back of rats (Fig. 6C). The thickness of the fibrous capsule wrapping the material is positively correlated with the degree of inflammatory response [49]. As shown in Fig. 6D and F, after 30 days of implantation,the fibrous capsule thickness exhibited a decreasing trend in the control group (109.45 ± 14.29 μm), the TA-Fe group (51.49 ± 7.58 μm), and the TA-Fe/rhCol I group (33.91 ± 6.05 μm), consistent with the results after 15 days of implantation. Furthermore, the immunofluorescence analysis (Fig. 6D, G, and 6H) suggested that the coating groups expressed more anti-inflammatory factor interleukin-10 (IL-10) and less proinflammatory factor tumor necrosis factor-α (TNF-α) than the control group after 15 and 30 days, indicating that the inflammation on the uncoated surface was more severe than that on the coated surfaces.
Polyphenols, natural anti-inflammatory agents, effectively regulate the activity of matrix metalloproteinases, while matrix metalloproteinases promote the invasion and activation of inflammatory cells[18,65,66]. Besides, as a principal component of ECM, rhCol I exhibits good histocompatibility and promotes ECM recovery [19,23]. These results indicated that the ECM-mimetic coating could modulate the inflammatory response by promoting the polarization of macrophages toward the anti-inflammatory M2 phenotype, improving the histocom patibility of materials.
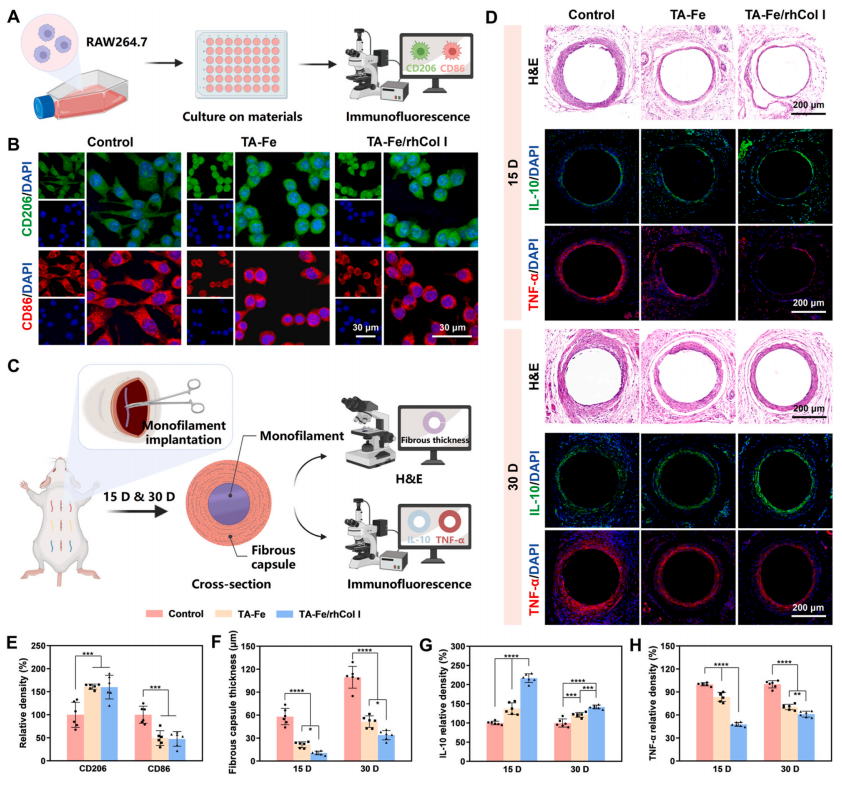
Fig. 6. Evaluation of inflammatory response. (A) Schematic of the macrophage experiment in vitro. (B) Immunofluorescence images and (E) quantitative statistics of CD206 and CD86 expression in RAW 264.7 cells after 2 days of culture on the sample surfaces. (C) Schematic of the subcutaneous implantation assay in the backs of rats. (D) Hematoxylin and eosin (H&E) and immunofluorescence images of the fibrous capsules wrapping different samples after 15 and 30 days of subcutaneous implantation, along with corresponding quantitative statistics of (F) fibrous capsule thickness, (G) IL-10 expression, and (H) TNF-α expression.
2.7. PDO monofilament implantation in the vessels
After implantation of the occluder in the heart, it is necessary to achieve rapid surface endothelialization and internal tissue regeneration, so as to achieve the purpose of blocking blood flow and sealing the defect [10,21]. We preliminary evaluated the effect of the ECM-mimetic coating on the occluder material in a pre-experiment, wherein a PDO monofilament, as the occluder skeleton material, was implanted into the rabbit’s carotid artery (Fig. 7A).
The expression of CD31 and endothelial nitric oxide synthase (eNOS) on the surface of PDO monofilaments was analyzed using immunofluorescence to evaluate the reendothelialization of the coating. CD31 exists at the tight junctions between ECs and is related to the mature phenotype of ECs [49,67]; eNOS is involved in nitric oxide (NO) production and is an important marker of endothelialization and the key to regulating vascular function [19,49]. High CD31 and eNOS expression indicates integrity and health of the endothelial layer. As shown in Fig. 7B–D and S9A, after 20 days of implantation, the bare PDO surface exhibited only a small amount of discontinuous expression of CD31(19.26 ± 5.30 %) and eNOS (34.71 ± 6.22 %). Compared to the bare PDO group, the expressions of CD31 (41.25 ± 2.96 %) and eNOS (52.40± 2.83 %) on the TA-Fe surface were significantly higher, while in the TA-Fe/rhCol I group, the densities of CD31 (72.47 ± 2.27 %) and eNOS(73.21 ± 2.39 %) further increased. After 40 days, the expression levels of CD31 and eNOS in all three groups enhanced to varying degrees;however, neither the bare PDO group nor the TA-Fe group achieved complete coverage of CD31 and eNOS, whereas the TA-Fe/rhCol I surface displayed a complete, continuous, and dense distribution of CD31 and eNOS, with significant differences still present among the three groups. These findings were consistent with the studies of EC growth behavior in vitro, and the TA-Fe/rhCol I surface achieved faster and more complete EC coverage, suggesting that the ECM-mimetic coating could accelerate the in vivo reendothelialization process.
Inflammatory response after occluder implantation may cause tissue erosion and rhythm disturbance, as well as hinder endothelialization and tissue healing [10,64]. In the vascular implantation model, persistent inflammation stimulates excessive neointimal hyperplasia, so the thicker neointima indicates a more serious inflammatory reaction [49].H&E staining of the vascular cross-sections (Fig. 7E and G, and S9B)showed that after 20 days, the neointimal areas in the bare PDO group(0.25 ± 0.03 mm2), TA-Fe group (0.16 ± 0.02 mm2), and TA-Fe/rhCol I group (0.11 ± 0.02 mm2) decreased significantly in turn. After 40 days of implantation, the neointimal area increased to 0.40 ± 0.04 mm2 in the bare PDO group, which was significantly higher than that in the TA-Fe group (0.32 ± 0.04 mm2), while that in the TA-Fe/rhCol I group was only 0.22 ± 0.02 mm2, much lower than both the PDO and TA-Fe groups. Therefore, the TA-Fe/rhCol I coating exhibited the least stimulation of intimal hyperplasia, demonstrating the lowest inflammatory response. In addition, the expression level of CD68, a marker of macrophages, reflects the degree of inflammation to a certain extent [68].The higher the expression of CD68, the more severe the inflammatory cell infiltration [27,49]. CD68 immunohistochemical analysis of the vascular cross-sections (Fig. 7F and H, and S9C) revealed that during the implantation period, CD68 density was highest in the bare PDO group,further decreased in the TA-Fe group, and was lowest in the TA-Fe/rhCol I group. This was consistent with the H&E results, indicating that the TA-Fe/rhCol I coating significantly suppressed the inflammatory response.
In summary, the ECM-mimetic coating modified on PDO monofilaments promoted reendothelialization and mitigated inflammation,creating a favorable environment for tissue healing. TA-Fe networks can inhibit platelet adhesion and activation by preventing plasma protein adsorption and denaturation on the material surface [41,47], while also suppressing inflammation-related matrix metalloproteinase activity and regulating macrophage polarization [18,65,66], providing an anti-thrombotic and anti-inflammatory platform suitable for endothelialization and tissue healing. Meanwhile, the tailored rhCol I with enhanced cell adhesion activity and good histocompatibility promotes proliferation, migration, and expression of functional proteins of ECs, accelerating the in vivo reendothelialization process. Hence, this ECM-mimetic coating has the potential for application in the surface modification of cardiac occluder.
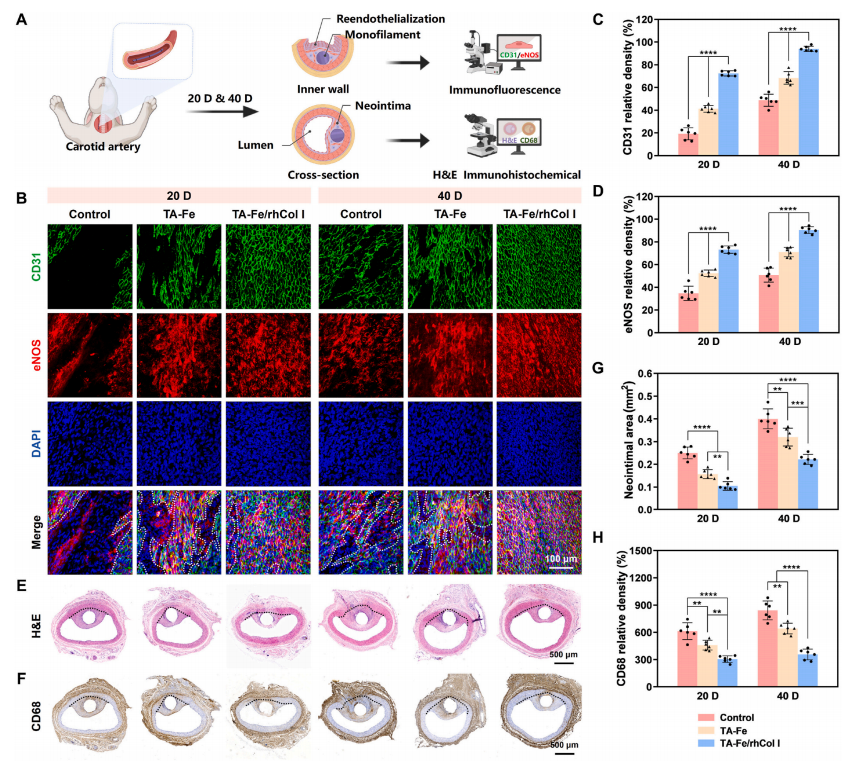
Fig. 7. Evaluation of reendothelialization and inflammation in PDO monofilament vascular implantation. (A) Schematic of the PDO monofilament implantation assay in the rabbit’s carotid artery. (B) Immunofluorescence images and (C, D) quantitative statistics of CD31 and eNOS expression on the bare, TA-Fe, and TA-Fe/rhCol I coated monofilament surfaces after 20 and 40 days of implantation. The non-endothelialized area is circled with white dashed line. (E) H&E and (F) immunohistochemical images of the vascular cross-sections containing the monofilaments after 20 and 40 days of implantation, along with corresponding quantitative statistics of (G) neointimal area and (H) CD68 expression. The black dashed line represents the boundary between the native vessel and the neointima.
2.8. Occluder implantation in a canine model
To further evaluate the effectiveness and safety of the ECM-mimetic coating applied to the occluder, the TA-Fe/rhCol I coated PDO occluders were implanted into the canine LAA (Fig. 8A). Transesophageal echocardiography (TEE) (Fig. 8C) and angiography (Fig. 8D) showed that the occluder was accurately positioned with a normal shape, and there was no residual shunt around the occluder. During implantation and followup, no abnormalities in cardiac function or morphology were observed(Fig. S10), no arrhythmias were detected on electrocardiogram (ECG)(Fig. S11), and routine blood tests, coagulation, and liver and kidney functions were all within normal ranges (Fig. S12). H&E staining of major organs after 3 months (Fig. S13) showed no significant histopathological lesions or abnormalities. These results indicated that the modified occluder was successfully implanted with a good occlusive effect and long-term safety.
Furthermore, to assess the in situ reendothelialization and tissue healing effects of the ECM-mimetic coating on the occluder, cardiac tissue containing the occluder was collected for histological evaluation after 3 months (Fig. 8B). SEM images of the occluder surfaces (Fig. 8F) showed that the ECs on the control surface were mostly irregular and randomly distributed, with only 64.04 ± 5.23 % EC coverage (Fig. 8E),whereas the TA-Fe/rhCol I coated surface was almost completely covered by dense and orderly ECs, achieving a more complete endothelialization (95.89 ± 4.31 %). Further, immunofluorescence analysis of endothelium-related proteins CD31 and eNOS on the occluder surfaces (Fig. 8G and H, and S14) indicated that compared to the control group, the expression levels of CD31 and eNOS were significantly higher in the TA-Fe/rhCol I group, indicating a higher degree of endothelialization. These results again demonstrated the ECM-mimetic coating’s ability to promote reendothelialization.
The acute inflammatory response raised by occluder implantation not only hinders the process of endothelialization, but also induces the risk of tissue erosion, which is detrimental to myocardial tissue repair.Besides, persistent inflammation may trigger rhythm disturbance, inducing transient arrhythmias [4,10]. The expression of macrophage phenotypic markers CD86 (M1) and CD206 (M2) reflects the degree of inflammation [27]. Immunofluorescence images of CD206 and CD86 expression (Fig. 8N) indicated that the coating group expressed more CD206 and less CD86, whereas the control group expressed the opposite.Statistical analysis (Fig. 8L) showed that the ratio of CD206 to CD86 in the coating group was markedly higher than that in the control group,suggesting that the TA-Fe/rhCol I coating could regulate the transformation of inflammatory cells to the anti-inflammatory M2 phenotype.
H&E, Masson’s trichrome, and Sirius red staining of the longitudinal section of the occluder were performed to evaluate the tissue repair effect (Fig. 8I). After 3 months, the occluder in the TA-Fe/rhCol I group was filled with dense and ordered fibrous tissue, whereas the center of the occluder in the control group was still partially uncovered by new tissue. Immunofluorescence analysis of CX43 expression (Fig. 8J and K) showed that CX43 density in the TA-Fe/rhCol I group was significantly higher than that in the control group, indicating that the TA-Fe/rhCol I coating promoted the expression of myocardium-related proteins. Vascular reconstruction is essential for myocardial tissue repair, as it can timely transport oxygen, nutrients, and cytokines to support cell growth[28,55]. CD31 and α-smooth muscle actin (α-SMA), as markers of vascular endothelium and muscle, respectively, are key indicators for evaluating angiogenesis [28]. The effect of the TA-Fe/rhCol I coating on angiogenesis in vivo was evaluated by immunofluorescence analysis of CD31 and α-SMA expression (Fig. 8M and O), which showed that the coating group exhibited more neovascularization than the control group. These findings demonstrated that the ECM-mimetic coating coordinated a well-organized endogenous regeneration process, promoting myocardial tissue healing.
Collectively, in the canine LAA occluder implantation model, the ECM-mimetic coating exhibited excellent ability of rapid reendothelialization. At the same time, the coating alleviated inflammation by modulating the macrophages to M2 polarization, and promoted the expression of the functional protein CX43 and angiogenesis, thereby facilitating myocardial repair. The anti-thrombotic and antiinflammatory TA-Fe networks, along with the highly cell-adhesive and low-inflammatory rhCol I, collectively respond to the complex physiological environment after occluder implantation (Fig. 8P). Hence, the ECM-mimetic coating optimizes the performance of the occluder and offers a novel perspective on functional coating design.
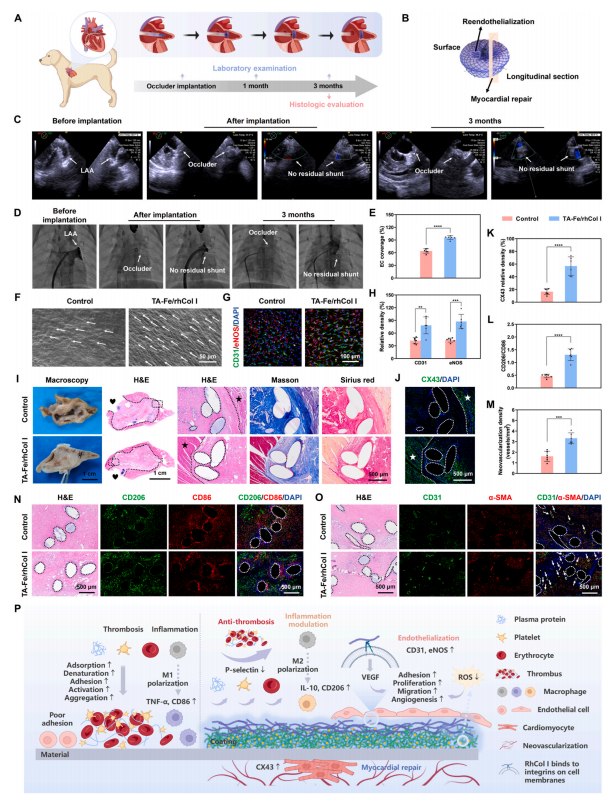
Fig. 8. Evaluation of reendothelialization and tissue healing in a canine occluder implantation model. (A) Schematic of the occluder implantation assay in the canine LAA. (B) Schematic of the histologic evaluation of the occluder. (C) TEE and (D) angiography images during occluder implantation and after 3 months. (E) Quantitative statistics of EC coverage and (F) SEM images on the uncoated and TA-Fe/rhCol I coated occluder surfaces after 3 months of implantation. (G) Immunofluorescence images and (H) quantitative statistics of CD31 and eNOS expression on the occluder surfaces. (I) Macroscopic views, and H&E, Masson’s trichrome, and Sirius red staining images of the longitudinal sections of the uncoated and TA-Fe/rhCol I coated occluders. (J) Immunofluorescence images and (K)quantitative statistics of CX43 expression in the longitudinal sections of the occluders. (L) Quantitative statistics of CD206 to CD86 ratio, and (N) H&E images and immunofluorescence images of CD206 and CD86 expression. (M) Quantitative statistics of angiogenesis, and (O) H&E images and immunofluorescence images of CD31 and α-SMA expression. (P) Schematic of the functional mechanisms of the TA-Fe/rhCol I coating. The love icon represents the left atrial chamber, the star marks the native myocardial tissue of the LAA, the dashed line indicates the boundary between the LAA and occluder, and the outline of the PDO monofilament is circled by the dashed line. The arrow in Fig. 8F represents the typical EC, and the arrow in Fig. 8O indicates neovascularization. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3. Conclusion
In this study, a customized rhCol I with a stable triple-helical structure based on the C1P1 motif was developed and used to construct a multifunctional ECM-mimetic coating for cardiac occluders. Briefly, TA and FeIII rapidly coordinated to form a stable network structure that effectively combined with rhCol I through multiple forces, thereby forming the ECM-mimetic TA-Fe/rhCol I coating. The rinsing experiments of FITC-conjugated rhCol I and QCM-D confirmed the stability of the coating structure. The metal-phenolic networks had dual anticoagulation and anti-inflammatory functions, and the tailored rhCol I exhibited high cell adhesion activity and low inflammatory response.Experiments demonstrated that the ECM-mimetic coating achieved a long-term anticoagulant effect, promoted the proliferation, migration, and angiogenesis of ECs, improved the functionality of cardiomyocytes, and suppressed the inflammatory response by polarizing inflammatory cells into an anti-inflammatory phenotype. Occluder implantation in the canine LAA model verified that the coating could accelerate the reendothelialization process and facilitate myocardial repair in vivo. All in all, the tailored ECM-mimetic coating addresses key clinical issues in thrombosis, inflammation, endothelialization, and tissue healing after occluder implantation, providing a promising surface modification strategy for advancing the development of cardiac occluders.
4. Materials and methods
4.1. Materials and reagents
Polydioxanone (PDO) sheets, monofilaments, and occluders were provided by Shanghai Shape Memory Alloy Co., Ltd. (Shanghai, China).Tannic acid (TA), Ferric(III) chloride hexahydrate (FeCl3⋅6H2O), trisbase buffer, phosphate-buffered saline (PBS), lactate dehydrogenase (LDH) cytotoxicity test kit, cell counting kit-8 (CCK-8), fluorescein diacetate (FDA), propidium iodide (PI), and fluorescein isothiocyanate (FITC) were purchased from Sigma-Aldrich (Shanghai, China). Ultrapure (UP) water, acquired through a Millipore purification system, was used in the experiments.
4.2. Preparation and identification of rhCol I
A total of six candidate peptides, named C1P1–C1P6, containing GER or GEK motifs, were selected from the triple-helical domain (179–1192aa) of the human COL1A1 protein (1–1464 aa). These peptides were synthesized using solid synthesis, as described in the Supporting Information (SI, M1). Subsequently, the pro-cell adhesion activity of these six
fragments was evaluated through HUVEC and H9C2 cell proliferation experiments (SI, M2). Among them, the C1P1 peptide fragment exhibited optimal cell-promoting effects and was selected. To obtain the amino acid sequence of rhCol I, this fragment was repeated four times in tandem.
The gene encoding the mentioned amino acid sequence was synthesized chemically after codon optimization. Subsequently, the recombinant expression vector containing the rhCol I gene was introduced into Escherichia coli for expression and translation into proteins after induction with isopropylthio-β-galactoside through fermentation engineering. The tailored rhCol I was subsequently prepared following further isolation and purification of the aforementioned proteins with nickel-chelate chromatography and anion-exchange chromatography.Crystallization and structure determination of human collagen type I peptide C1P1S were described in SI, M3, and rhCol I quantification was performed, as shown in SI, M4.
4.3. Preparation of the ECM-mimetic coating
To ensure data collection reliability, identical coatings were applied to various substrates, including PDO sheets, silicon wafers, gold-plated quartz crystals, PDO monofilaments, and PDO occluders, for the corresponding material and biological characterization. Briefly, substrates were rapidly immersed in a freshly mixed aqueous solution of TA (1 mg/mL, pH 4.0) and FeCl3⋅6H2O (0.2 mg/mL, pH 3.2) at 25◦C for 5 min,followed by washing with ultrapure water to eliminate poorly adsorbed components. After repeating this process five times, coatings of metalphenolic networks were obtained and denoted as TA-Fe. Subsequently, the TA-Fe coated substrates were immersed in a 2 mg/mL rhCol I solution prepared with tris-base buffer (10 mM, pH 8.0) at 25◦C for 8 h and thoroughly washed with ultrapure water. The product was denoted as TA-Fe/rhCol I. Bare substrates without coatings were used as controls.
4.4. Characterization of the ECM-mimetic coating
The surface morphology was measured using FE-SEM (Apreo S HiVac, Thermo Fisher Scientific, USA), and the elemental distribution was analyzed using EDS. Surface roughness was determined using AFM(MFP-3D-BIO, Asylum Research, USA). The thickness of the coating on the silicon wafer was measured using an ellipsometer (UVISEL, HORIBA Scientific, Japan). The chemical structure and elemental composition of the coating were determined using ATR-FTIR spectroscopy (SpectrumTWO, PerkinElmer, USA) and XPS (AXIS Ultra DLD, Kratos, UK). Additionally, QCM-D (QSense Analyzer, Biolin Scientific, Sweden) was used to monitor the coating assembly process in real time (SI, M5) [49,69].Surface hydrophilicity and zeta potential were measured using a WCA measuring instrument (Attension Theta, Biolin Scientific, Sweden) and a solid surface zeta potential analyzer (SurPASS 3, Anton Paar, Austria),respectively.
4.5. Stability test of the ECM-mimetic coating
To evaluate the stability of the coating, FITC-conjugated rhCol I was prepared (SI, M6) and used to construct the TA-Fe/rhCol I coating according to the above method [19]. The FITC-conjugated TA-Fe/rhCol I coating was immersed in PBS which was refreshed every 2 days, and placed on a shaker at 75 rpm and 25◦C under dark conditions. The fluorescence intensity of the coating was monitored by CLSM (ZEISS LSM880, Carl Zeiss, Germany) after immersion in PBS for 0, 7, and 14 days, and quantified by ImageJ software (set the initial fluorescence intensity at 0 day to 100 %).
4.6. RhCol I quantification
The rhCol I loading in the TA-Fe/rhCol I coating was quantified using BCA assay (P0010S, Beyotime, China) [39]. Initially, the standard curve was prepared according to the instructions in the manual, as detailed in SI, M7. The TA-Fe/rhCol I coated PDO occluders were soaked in a mixture (v/v 1/10) of PBS and BCA working solution at 25◦C for 2 h.Subsequently, the absorbance of the reaction solution was measured at 562 nm using a microplate reader (Synergy H1, BioTek Instruments, Inc.USA). The loading density of rhCol I was calculated based on the standard curve. In addition, in order to further determine the stability of rhCol I in the coating, the TA-Fe/rhCol I coated PDO occluders were immersed in PBS which was refreshed every 2 days, and placed on a shaker at 75 rpm and 25◦C for 7 and 14 days. The density of rhCol I in the coating at different time points was measured according to the aforementioned BCA method.
4.7. Static platelet and whole blood adhesion
Fresh rabbit whole blood was collected with sodium citrate (v/v 9/1), and platelet-rich plasma (PRP) was obtained by centrifuging fresh whole blood at 1500 rpm for 15 min and collecting the supernatant. Subsequently, the bare, TA-Fe, and TA-Fe/rhCol I coated PDO monofilaments (diameter, Φ, 300 μm) were incubated with PRP and whole blood at 37◦C for 1 h. Following PBS rinse and 2.5 % glutaraldehyde fixation for 12 h, platelets and erythrocytes adhering to the surfaces were observed by SEM. Platelet count and quantification utilized ImageJ software and the LDH cytotoxicity test kit (SI, M8) [70]. Freshly prepared samples were immersed in PBS which was refreshed every 2 days, and placed on a shaker at 75 rpm and 25◦C for 7, 14, and 28 days. The platelet adhesion and activation on the sample surfaces were examined using SEM to verify the long-term anticoagulant performance of the coating. In addition, immunofluorescence analysis of P-selectin, a specific marker of platelet activation [50], was performed to assess the degree of platelet activation on the surface of each sample, as described in SI, M9.
4.8. Ex vivo thrombogenicity assay
All animal experiments were approved by the Medical Ethics Committee of Sichuan Provincial Committee for Experimental Animal Management and followed the Guidelines for the Care and Use of Laboratory Animals of the Sichuan University (No. KS2020394). Male New Zealand white rabbits (2.8–3.0 kg, Dossy, Chengdu, China) were used to establish an arteriovenous shunt model for an ex vivo blood circulation experiment [27,49]. Under anesthesia, the left carotid artery and right jugular vein were separated from the neck of the rabbit and connected to commercial polyvinylchlorid catheters, with sterilized PDO flakes tightly attached to the inner walls to establish a circulation loop for 2 h. Subsequently, the samples were collected and rinsed with PBS. The occlusion rate of the catheter was calculated using ImageJ software, and the sample surface was observed using SEM after immobilization and dehydration.
4.9. Protein adsorption
BSA-FITC and FBG-FITC were purchased from Beijing Solarbio Science & Technology Co., Ltd. (Beijing, China). Briefly, the bare, TA-Fe,and TA-Fe/rhCol I coated PDO sheets were incubated with BSA-FITC(1 mg/mL) and FBG-FITC (0.1 mg/mL) diluted with PBS at 37◦C for2 h in the dark. After rinsing with PBS, proteins adsorbed onto the surfaces were quantified using a microplate reader, exciting at 488 nm and emitting at 525 nm, and qualitatively observed using an inverted fluorescence microscope (NIB900-FL, Nexcope, USA).
4.10. Hemolysis assay
Fresh rabbit whole blood was centrifuged at 1500 rpm for 15 min,and the erythrocyte precipitate at the bottom was collected and diluted 50 times (2 %) with PBS. The bare, TA-Fe, and TA-Fe/rhCol I coated PDO sheets were incubated with 2 % erythrocyte dilution at 37◦C for 1 h.Erythrocytes diluted with ultrapure water served as the positive control(+), while those diluted with PBS served as the negative control (− ).After co-incubation, the incubation solution was centrifuged at 3000rpm for 5 min, and the absorbance (OD value) of each supernatant was measured at 540 nm using a microplate reader. The hemolysis rate was calculated using the following formula: Hemolysis (%) = (ODsample –ODnegative)/(ODpositive – ODnegative) × 100 %.
4.11. HUVECs behavior assessment
HUVECs (Otwo Biotech, Shenzhen, China) were cultured in endothelial cell medium (ScienCell Research Laboratories, USA) containing 10 % fetal bovine serum (FBS) and 1 % penicillin/streptomycin (PS) at 37◦C under a 5 % CO2 atmosphere. Survival, proliferation, migration,tube formation, and VEGF (GB111971, Servicebio, China) expression of HUVECs were studied and details were described in SI, M10.
4.12. H9C2 cell behavior assessment
Rat cardiomyocytes H9C2 (Procell, Wuhan, China) were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Gibco, ThermoFisher Scientific, USA) containing 10 % FBS and 1 % PS at 37◦C under a 5 % CO2 atmosphere. Survival, proliferation, morphology, and CX43 expression of H9C2 cells were studied and details were described in SI,M11.
4.13. In vitro macrophages behavior assessment
Mouse mononuclear macrophage leukemia cells RAW 264.7 (West China Hospital, Chengdu, China) were cultured in high-glucose DMEM (Gibco, ThermoFisher Scientific, USA) containing 10 % FBS and 1 % PS at 37◦C under a 5 % CO2 atmosphere. The expressions of macrophage phenotypic markers CD86 and CD206 in RAW264.7 cells were studied and details were described in SI, M12.
4.14. In vivo subcutaneous implantation assay
Male Sprague-Dawley rats (200–220 g, Dossy, Chengdu, China) were used for the subcutaneous implantation assay to assess in vivo inflammatory response [27,49]. The sterilized bare, TA-Fe, and TA-Fe/rhCol I coated PDO monofilaments (Φ 300 μm, length 10 mm) were implanted subcutaneously into the back of rats (three on each side) under anesthesia. After 15 and 30 days, the hyperplastic fibrous tissues around the samples were collected, and H&E (G1005, Servicebio, Wuhan, China) staining, as well as immunofluorescence staining with anti-IL-10 antibody (GB11108, Servicebio, Wuhan, China) and anti-TNF-α antibody (GB11188, Servicebio, Wuhan, China) in cross-sections, were carried out. The fibrous capsule thickness and the relative densities of IL-10 and TNF-α were calculated using ImageJ software (set the control to 100 %).
4.15. Intravascular implantation assay of PDO monofilament
Male New Zealand white rabbits (2.4–2.6 kg) were used for the intravascular implantation assay of PDO monofilament to simulate the direct contact between the occluder skeleton and the endocardium.Under anesthesia, the sterilized bare, TA-Fe, and TA-Fe/rhCol I coated PDO monofilaments (Φ 300 μm, length 20 mm) were implanted into the rabbit’s carotid artery and tightly fixed to the inner wall of the vessel with sutures. After 20 and 40 days, vessels containing the PDO monofilaments were collected, and the growth of ECs on the surface of the monofilaments was observed by immunofluorescence co-staining with anti-CD31 (ab9498, Abcam, UK) and anti-eNOS (ab5589, Abcam, UK) antibodies. Furthermore, cross-sections were stained with H&E and antiCD68 antibody (Invitrogen, Carlsbad, CA, USA) to evaluate histocompatibility. Corresponding statistics were calculated using ImageJ software, with the healthy rabbit’s carotid artery as the reference(set to100 %).
4.16. Occluder implantation assay
Female Labrador dogs (20–22 kg, Jiagan, Shanghai, China) were used for the occluder implantation assay. Under anesthesia, the sterilized bare and TA-Fe/rhCol I coated PDO occluders (sealing disc Φ 22.0± 2.5 mm, fixed disc Φ 16.0 ± 2.5 mm, fixed disc height 6.0 ± 2.5 mm)were implanted into the canine LAA through the right femoral vein and atrial septal puncture with the guidance of angiography and TEE (SI,M13). Before and after implantation, as well as at 1 and 3 months of follow-up, ECG and hematological tests were performed. After 3 months,the occlusive effect was examined using angiography and TEE, and cardiac tissues containing the occluders were collected and fixed.Endothelialization on the occluder surface was observed using SEM and immunofluorescence co-staining with anti-CD31 and anti-eNOS antibodies. Longitudinal sections were stained with H&E, Masson’s trichrome, Sirius red, anti-CX43 (13–8300, Invitrogen, USA), anti-CD31/anti-α-SMA (ab7817, Abcam, UK), and anti-CD206 (24595, CST, USA)/anti-CD86 (ab220188, Abcam, UK) antibodies to evaluate myocardial tissue repair and histocompatibility. Corresponding statistics were calculated using ImageJ software, with the healthy canine cardiac tissue as the reference (set to 100 %).
4.17. Statistical analysis
All experiments were repeated at least thrice. The data were
analyzed using ImageJ and GraphPad Prism 8.0 softwares. All experimental data were analyzed by two investigators in blinded and the results were expressed as mean ± standard deviation (SD). The quantilequantile plot was used to assess the normality and variance before statistical analysis by Brown-Forsythe and Bartlett’s tests. Differences between the two groups were determined using independent samples ttests or nonparametric tests. Grouped data were analyzed using one-way ANOVA followed by Tukey’s post hoc test or two-way ANOVA with Sidak’s multiple comparisons test. Statistical significances were represented by *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001.Error bars were defined as SD.
CRediT authorship contribution statement
Yumei Qin: Writing – original draft, Visualization, Validation,Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Yun Zhu: Visualization, Methodology, Formal analysis, Data curation. Lu Lu: Formal analysis, Validation. Haoshuang Wu: Validation, Formal analysis. Jinpeng Hu: Formal analysis, Validation. Fan Wang: Formal analysis, Validation. Bo Zhang: Methodology, Formal analysis, Writing – review & editing. Jian Wang: Formal analysis,Validation. Xia Yang: Formal analysis, Validation. Rifang Luo: Methodology, Formal analysis, Writing – review & editing. Juan Chen:Y. Qin et al. Biomaterials 313 (2025) 12276915Formal analysis, Validation. Qing Jiang: Supervision, Resources,Funding acquisition. Li Yang: Writing – review & editing, Supervision,Resources, Funding acquisition, Conceptualization, Methodology.Yunbing Wang: Writing – review & editing, Supervision, Resources,Project administration, Funding acquisition, Conceptualization. Xingdong Zhang: Supervision, Resources, Project administration.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability
Data will be made available on request.
Acknowledgments
This work was supported by the National Key Research and Development Programs, China (2023YFC2412700 and 2023YFC2412704),the National Natural Science Foundation of China (32101107), and the CAMS Innovation Fund for Medical Sciences (2021-I2M-5–013). We thank Li Li, Fei Chen and Chunjuan Bao (Institute of Clinical Pathology,West China Hospital) for processing histological staining.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.biomaterials.2024.122769.
扫码观看可降解学苑精彩内容
医谱app
扫码或者点击图片下载
微信公众号
扫码或点击图片关注
版权及免责声明:
本网站所发表内容知识产权归属医谱平台、主办方以及原作者等相关权利人,未经许可,禁止进行复制、传播、展示、镜像、上载、下载、转载、摘编等。经授权使用,须注明来源,否则将追究其法律责任。有关作品内容、版权和其他问题请与本网联系。




发表留言
暂无留言
输入您的留言参与专家互动