Boston Scientific's FARAPULSE™ series of pulsed electric field ablation products received NMPA approval

Atrial fibrillation (AF) is one of the most common rapid arrhythmias, severely impacting patients' quality of life and significantly increasing risks of death, stroke, and heart failure. According to extensive epidemiological surveys, there are approximately 12 million AF patients in China currently [1]. With the ongoing aging of the population, it is expected that the number of AF patients will further increase in the future, posing a substantial burden on individuals and families and becoming a serious public health issue threatening people's health.
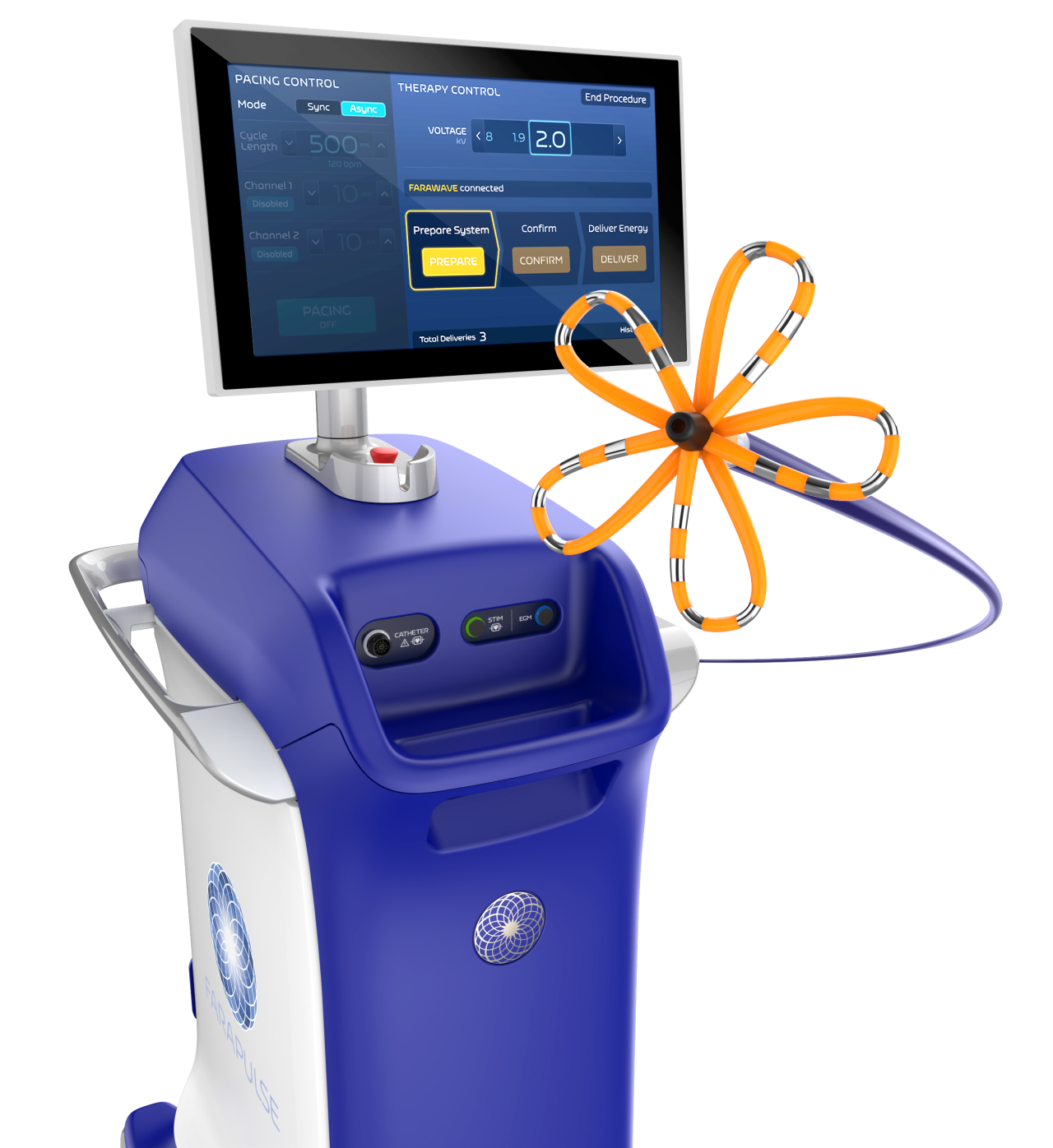
Currently, catheter ablation has gradually become a frontline treatment for rhythm control of AF [2]. Existing ablation methods primarily include radiofrequency ablation and cryoablation. The novel Pulsed Field Ablation (PFA) technology selectively ablates cardiac tissue using pulsed electric fields, reducing the risk of damage to adjacent tissues such as the esophagus. The unique design of the FARAWAVE ablation catheter allows operators to flexibly adjust it into petal and net configurations during procedures, facilitating adaptation to the patient's pulmonary vein morphology to achieve pulmonary vein isolation. Its optimized energy release pattern helps reduce the risk of complications associated with thermal effects during ablation.
Key findings from the pivotal ADVENT study (the first randomized controlled trial directly comparing the effectiveness and safety of FARAPULSE series PFA products with traditional thermal ablation) at 12 months demonstrate that the FARAPULSE series PFA products are non-inferior in safety and efficacy compared to traditional thermal ablation, with significantly reduced ablation times [3]. Additionally, real-world data from the MANIFEST-17K registry study involving over 17,000 patients have not reported incidents of permanent phrenic nerve paralysis, pulmonary vein stenosis, or esophageal injury [4].
In line with the vision of "simultaneous global innovation achievements launching in China," Boston Scientific continues to accelerate the introduction of the FARAPULSE series products to benefit domestic patients. Leveraging policies such as the "Innovative Pathway," "First-In-Human Trials," and "Hong Kong-Macao Medical Device Access," this system has already been deployed clinically in the Lecheng area of Hainan and the Greater Bay Area ahead of others.
Zhang Jun, President of Boston Scientific Greater China, stated: "Meeting the diverse diagnostic and therapeutic needs of Chinese patients has always been Boston Scientific's unwavering focus. The approval and market launch of the FARAPULSE series products exemplify our capability in leveraging cutting-edge global innovations to support the development of the healthcare industry in China. Looking ahead, we will continue to introduce innovative minimally invasive intervention solutions, bridging gaps in diagnosis, treatment, and application across various disease areas in China with advanced technologies, thereby promoting high-quality development in the healthcare industry."
[1] 中华心血管病杂志, 2023,51(6) : 572-618. DOI: 10.3760/cma.j.cn112148-20230416-00221.
[2] 中华心血管病杂志, 2023,51(6) : 572-618. DOI: 10.3760/cma.j.cn112148-20230416-00221.
[3] Reddy VY, Gerstenfeld EP, et al. Pulsed Field or Conventional Thermal Ablation for Paroxysmal Atrial Fibrillation. N Engl J Med. 2023 Aug 27. doi: 10.1056/NEJMoa2307291. Epub ahead of print. PMID: 37634148.
[4] Reddy VY, Ekanem E, et al., Multi-national survey on the safety of the post-approval clinical use of pulsed field ablation in 17,000+ patients. (MANIFEST-17K). AHA 2023.

发表留言
暂无留言
输入您的留言参与专家互动