EuroPCR 2024 | Professor Song Lei: 12-Month Randomized Controlled Clinical Results of IBS Iron-Based Bioresorbable Scaffold Equivalent to Co-Cr DES — Interpretation of the IRONMAN-II Study
On May 14, 2024, during the Hotline session at EuroPCR 2024, Professor Song Lei from Fuwai Hospital, Chinese Academy of Medical Sciences, representing the research team of Academician Gao Runlin, announced the 1-year clinical results of the IRONMAN-II randomized controlled study. The IRONMAN-II study primarily evaluated the safety and efficacy of Element Cardiovascular Technology's next-generation ultra-thin (strut thickness approximately 55μm) iron-based bioresorbable scaffold (IBS). We are honored to invite Professor Song Lei to interpret this study.
Background of the Study:
IBS is an ultra-thin sirolimus-eluting iron-based bioresorbable scaffold. Preliminary studies have shown that IBS does not degrade in the body of pigs within 6 months and completes full degradation in 2-3 years. Additionally, its endothelial coverage rate is faster than that of Co-Cr DES stents. In the FIM study, the TLF (target lesion failure) rate in the IBS group was 2.2% at 6 months and 6.7% at 3 years, with no occurrences of death, myocardial infarction, or thrombotic events. In order to further confirm the safety and efficacy of IBS stents, the IRONMAN-II randomized controlled trial was formally initiated.
Study Design:
The IRONMAN-II study is a prospective, multicenter, single-blinded, randomized controlled trial. It primarily includes CAD patients aged 18-75 years old. The main inclusion criteria include patients with evidence of myocardial ischemia suitable for elective PCI surgery, 1-2 primary target lesions not in the same vessel, with a stenosis severity of ≥70% (or ≥50% with evidence of myocardial ischemia in this range), and visually assessed target lesion length ≤33 mm and reference vessel diameter (RVD) of 2.5-4.0 mm, among others. Patients are randomly assigned in a 1:1 ratio to either the IBS stent group or the Xience stent group. Clinical follow-up visits are scheduled at 1 month, 6 months, and 1 year post-surgery, followed by annual follow-ups up to 5 years, with angiographic reassessment at 2 years post-surgery.
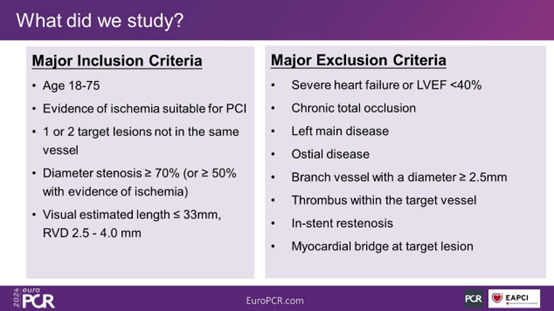
Figure 1. Inclusion and Exclusion Criteria of the Study
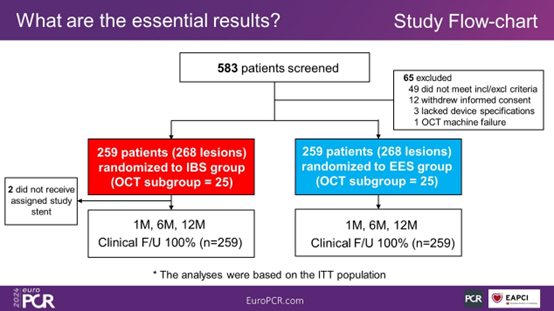
Figure 2. Study Flowchart
Study Endpoints:
The primary endpoint of this study is the in-segment late lumen loss (LLL) at 2 years post-surgery. The major secondary endpoints include the quantitative flow ratio (QFR) at 2 years post-surgery and the measurement of mean lumen area at the cross-sectional level using optical coherence tomography (OCT) (only in the OCT subgroup). Other secondary endpoints comprise the target lesion failure (TLF) rate at each follow-up point, patient-oriented composite endpoint (PoCE), target lesion revascularization (TLR), target vessel revascularization (TVR), stent thrombosis, among others.
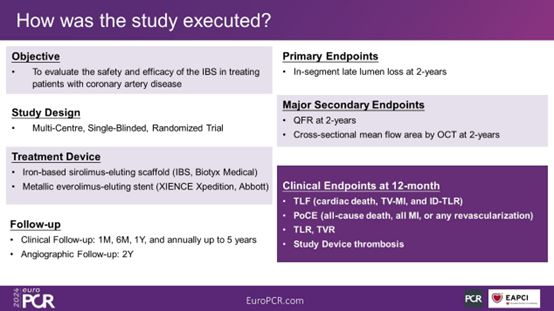
Figure 3. Study Endpoints
Study Results:
The IRONMAN-II study ultimately enrolled a total of 518 patients.
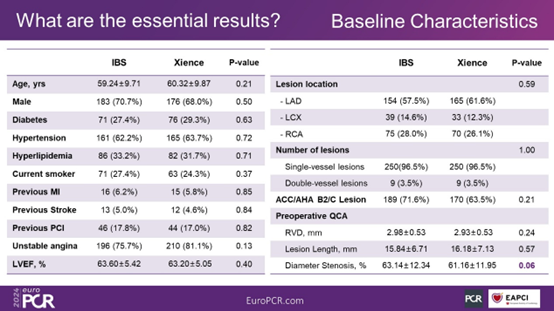
Figure 4. Baseline Characteristics
The results show that the technical success rate and lesion success rate were both 100% in both the IBS group and the Xience group. The clinical success rate was 99.6% in the IBS group and 98.8% in the Xience group (P = 0.62).
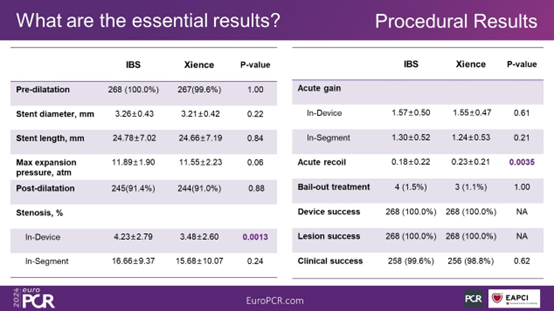
Figure 5. Procedural Outcomes
Clinical follow-up results at 1 year post-surgery show that the TLF occurrence rates in the IBS group and the Xience group were 2.3% and 2.7%, respectively (P = 0.78). In the IBS group, there were no occurrences of cardiac death. The rates of PoCE in the two groups were 5.0% and 4.6%, respectively (P = 0.48). There were no occurrences of stent thrombosis in either group.
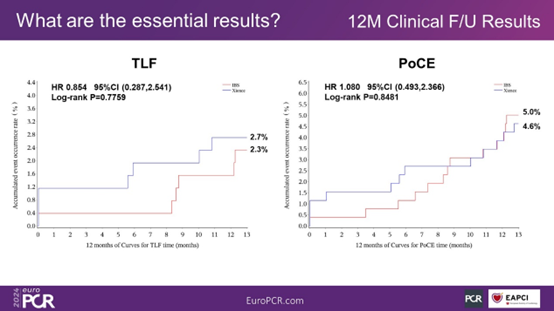
Figure 6. TLF and PoCE at 1 Year Post-Surgery
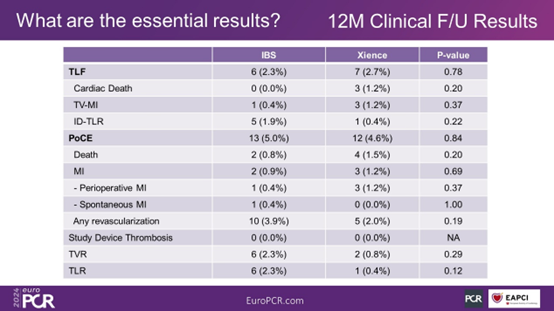
Figure 7. Clinical Outcomes at 1 Year Post-Surgery
Researchers' Interpretation:
"One intervention without implantation" is one of the goals pursued by interventional cardiologists, and both clinicians and researchers hope that bioresorbable scaffolds (BRS) can become a breakthrough in the field of coronary intervention therapy. Previous studies have shown that PLLA-based BRS increases the incidence of thrombosis and myocardial infarction during scaffold absorption, and the withdrawal of Absorb BVS has prompted people to think about the reasons for the high incidence of events such as thrombosis formation. What role do scaffold materials and technical operations play in this? Meanwhile, researchers at home and abroad are still making unremitting efforts to find better materials or techniques to achieve the goal of "one intervention without implantation."
Iron-based bioresorbable scaffold (IBS) is a new type of sirolimus-eluting scaffold. It is designed as a composite material by "surface treatment + alloying" of iron nitride alloy, optimizing the comprehensive performance of the material. The scaffold matrix is made of iron nitride obtained by plasma nitriding, and zinc is uniformly deposited on the surface of iron nitride through electroplating, followed by ultrasonic atomization spraying of PDLLA drug-eluting coating. Previous studies have shown that the scaffold exhibits good cell compatibility and blood compatibility, with a low incidence of TLF.
The results of the IRONMAN-II study released this time are encouraging: at 12 months, the TLF rate in the IBS stent group was 2.3%, the PoCE rate was 5.0%, and there were no cardiac deaths or scaffold thrombosis events, indicating that IBS has good safety and efficacy. Compared with the Xience stent, which is known as the "gold standard," there were no statistically significant differences in various study endpoints between the two groups.
Overall, the 1-year results of the IRONMAN-II study support the potential of IBS in clinical application, with its bioresorption characteristics and lower incidence of adverse events providing a new direction for future coronary artery disease intervention therapy. The study is still ongoing, and data related to the primary endpoint - in-segment late lumen loss (In-segment LLL) - will be released after 1 year. In addition, the IRONMAN-III single-group target value study is also underway, which will provide more evidence-based research on IBS in the future.

发表留言
暂无留言
输入您的留言参与专家互动