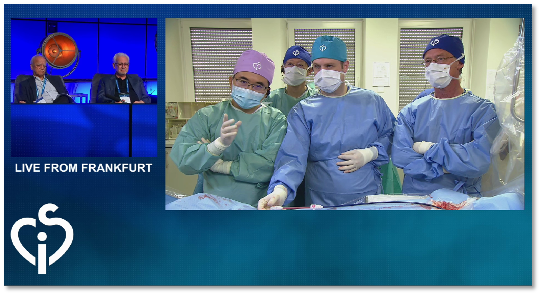
CSI Frankfurt 2024, Professor Xiangbin Pan demonstrates the procedure of biodegradable PFO occluder under ultrasound-guided intervention
In September 2023, the world's first biodegradable patent foramen ovale (PFO) occluder was officially approved and launched, marking a significant advancement in the prevention and treatment of cardioembolic strokes in the field of structural heart disease.
Recently, Professor Pan Xiangbin from Fuwai Hospital, Chinese Academy of Sciences, traveled to Germany upon the invitation of the CSI Frankfurt 2024 and successfully performed a procedure using an ultrasound-guided biodegradable PFO closure device.
Patient Profile
Sex: Female
Age:56 years old
Present Medical History: CT scan indicates old ischemic stroke (excluding other potential causes except PFO)
Past Medical History: Nickel allergy
Examinations:
-
Electrocardiogram (ECG): Normal
-
Holter monitoring: Normal
-
CT/MRI: Old ischemic lesions
-
Transthoracic echocardiography (TTE): Norma
Clinical Strategy
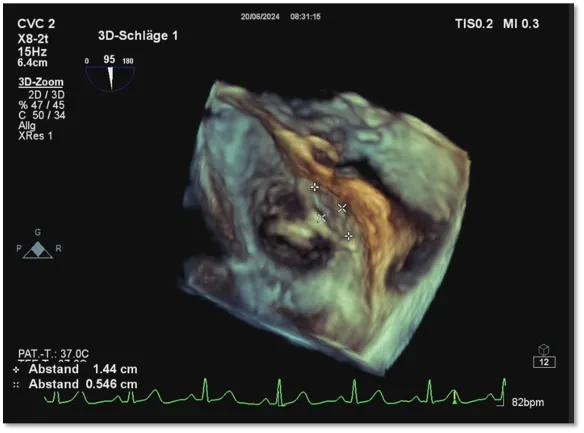
Defect Characteristics: The tunnel measures 14.4mm in length and 5.46mm in width.
Device Selection Considerations: Based on the defect characteristics, the patient presents with a large, long-tunnel PFO. During the foam test performed intraoperatively, a significant amount of bubbles were observed in the left heart. Therefore, the recommended choice is the symmetric PFO occluder, BDPFO-I 3434, with a 14F biodegradable sheath
Preoperative Strategy: The procedure will be conducted via the femoral approach, guided by transesophageal echocardiography for crossing the PFO and releasing the biodegradable PFO occluder. Additionally, the Pannawire will be used for wire guidance during the procedure.
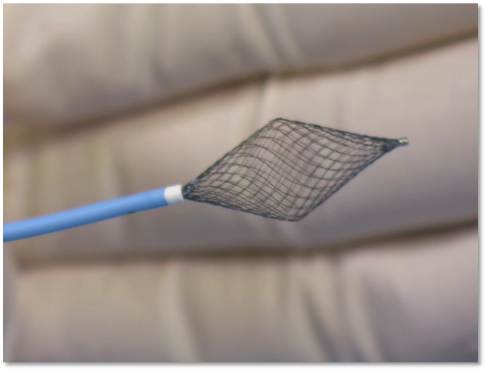
Pannawire guidewire
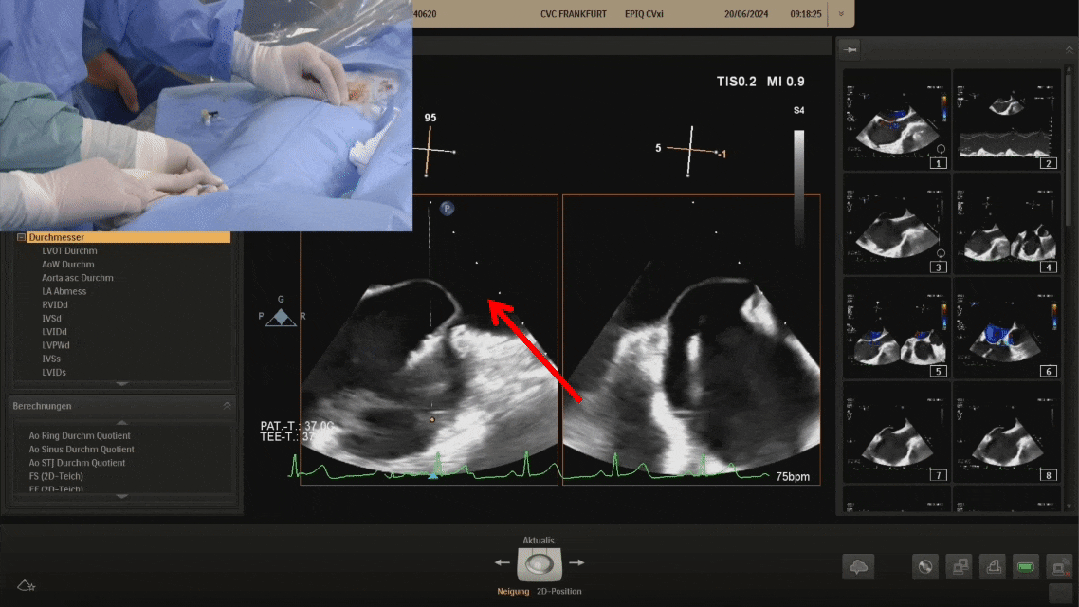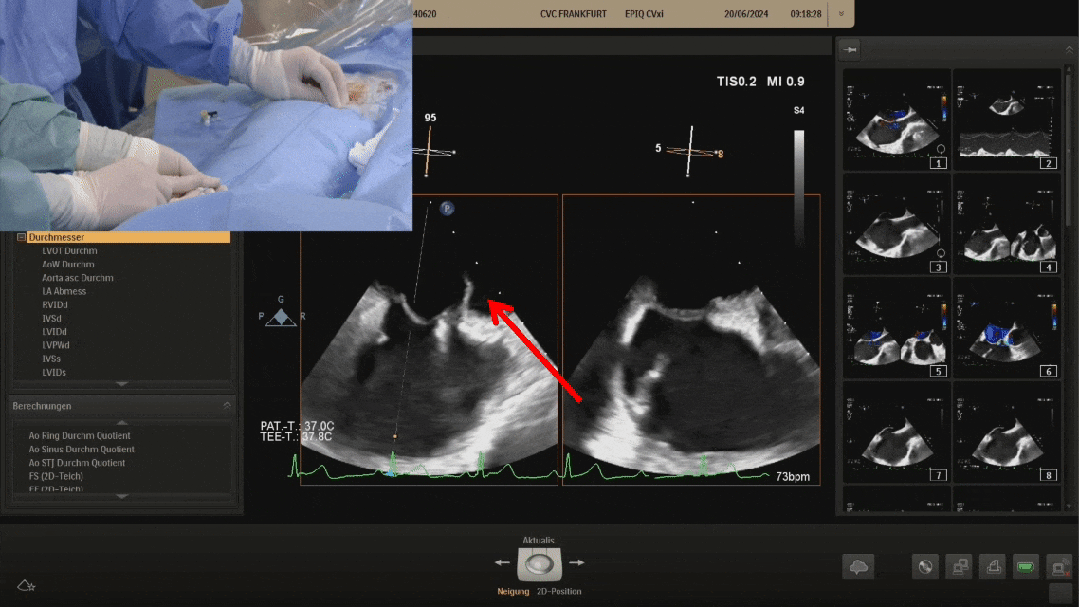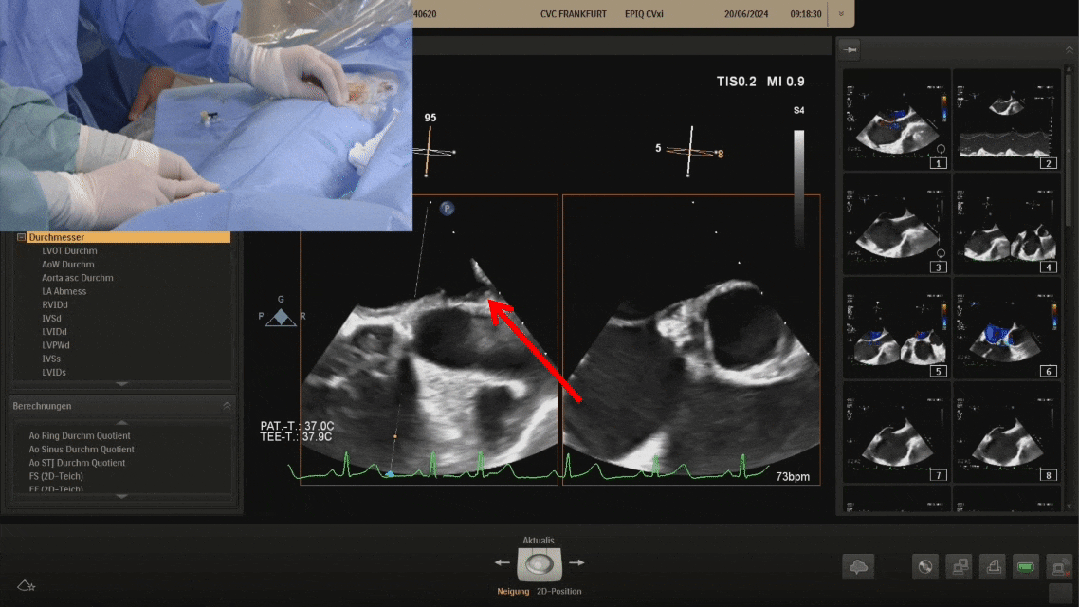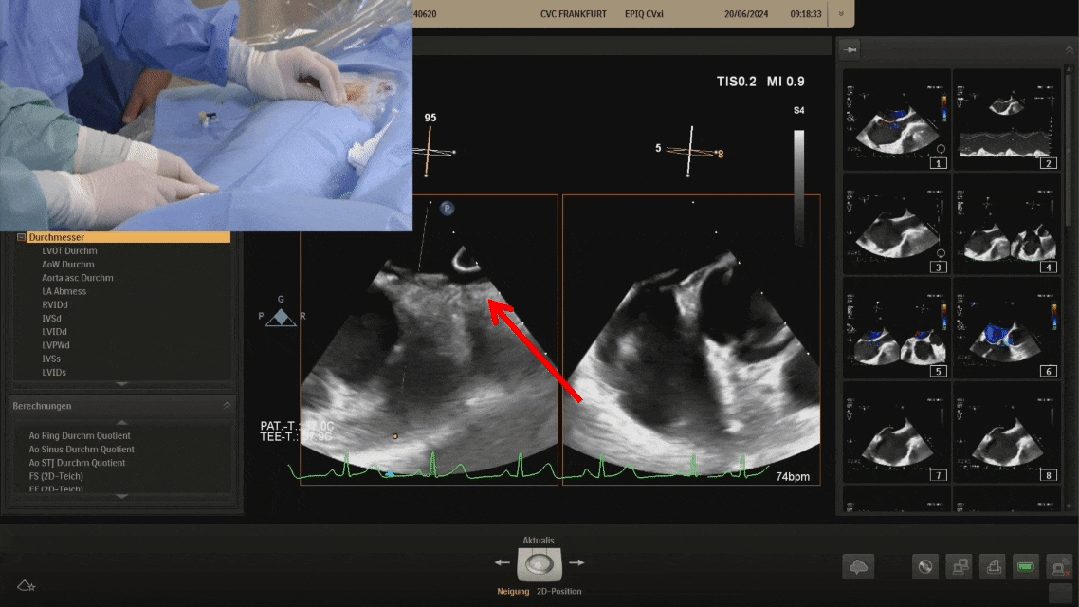
Pannawire guiding wire crossing the PFO
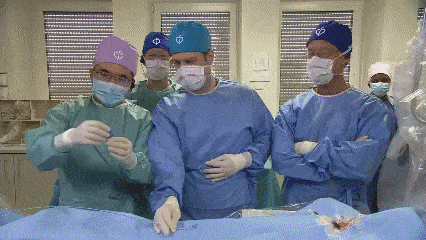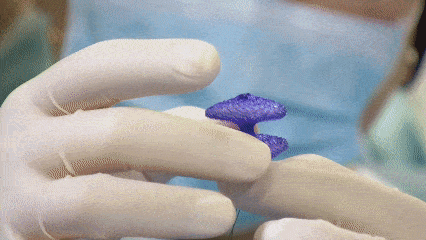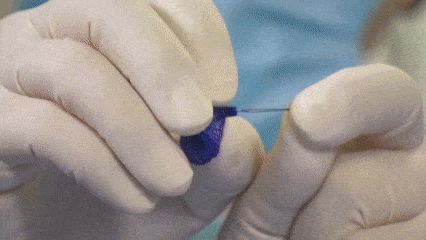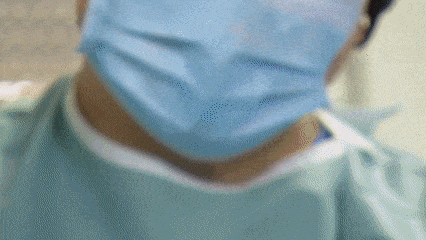
Display of products during the procedure

Loading the occluder during the procedure
Delivery sheath reaching the left atrium
Left disc release

”Forward-pushing steel cable to release the left disc. During the release process, the left disc presents a "lantern shape"
Pulling the shaping wire to shape the left disc, retracting the steel cable to make the left disc fit snugly against the septum
Release of the right disc
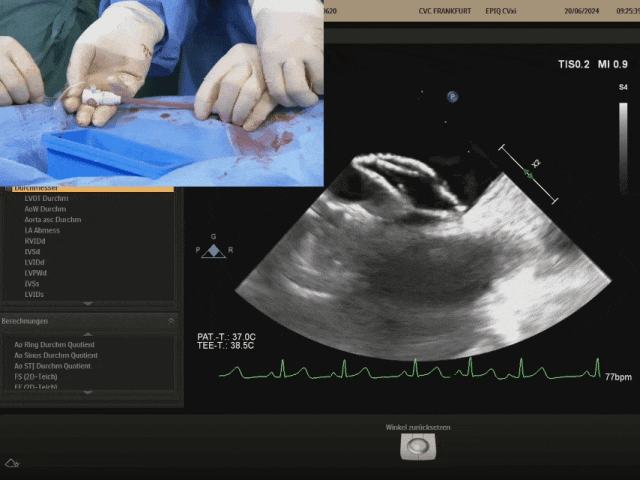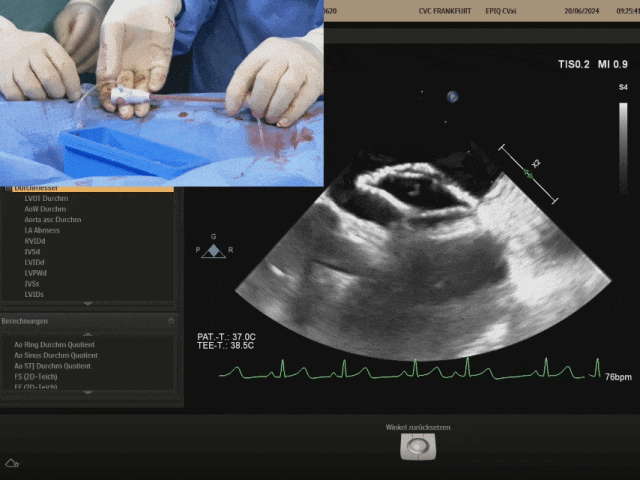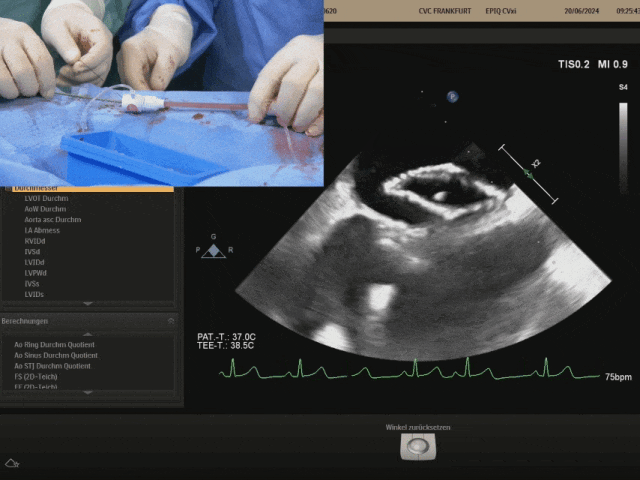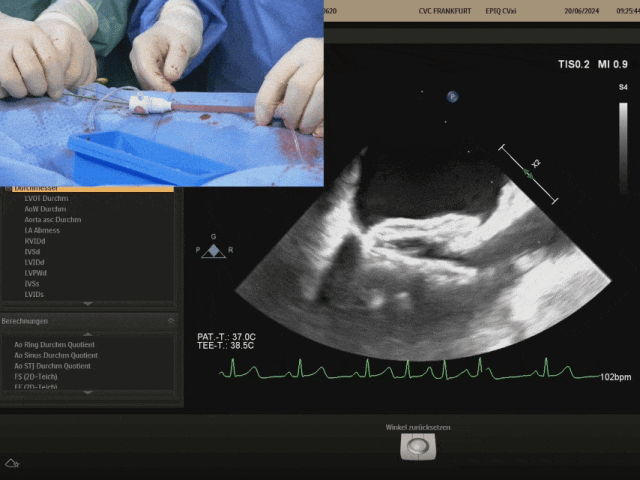
Retracting the sheath slowly to fully expose the right disc. After the right disc is fully exposed, gently push the steel cable and gently pull the shaping wire to shape the occluder
Before locking, confirm whether the occluder shape is appropriate
Pulling the steel cable and delivery system, the right disc swings with the septum
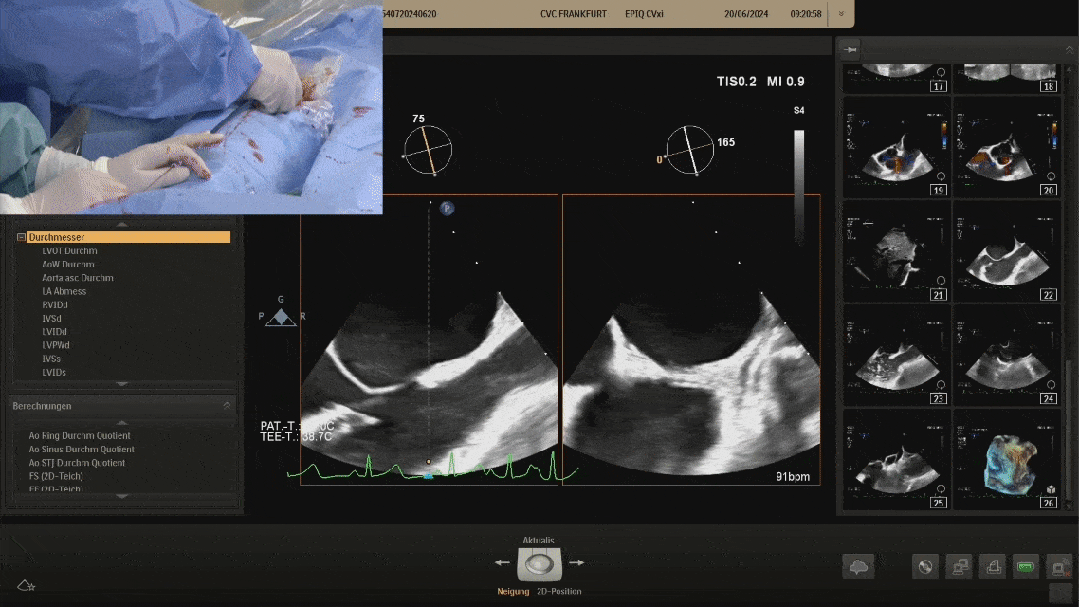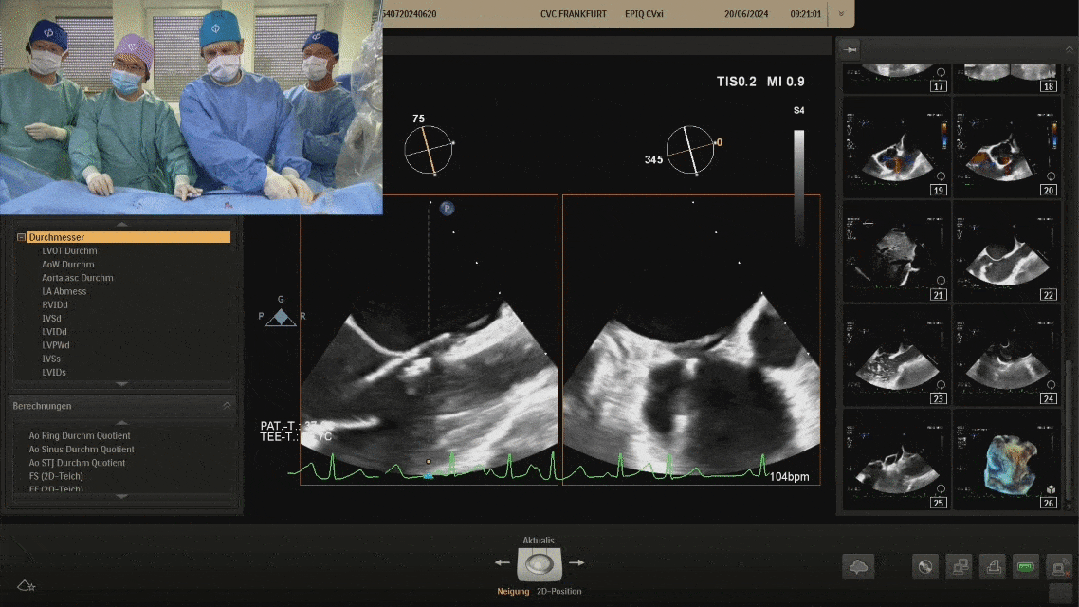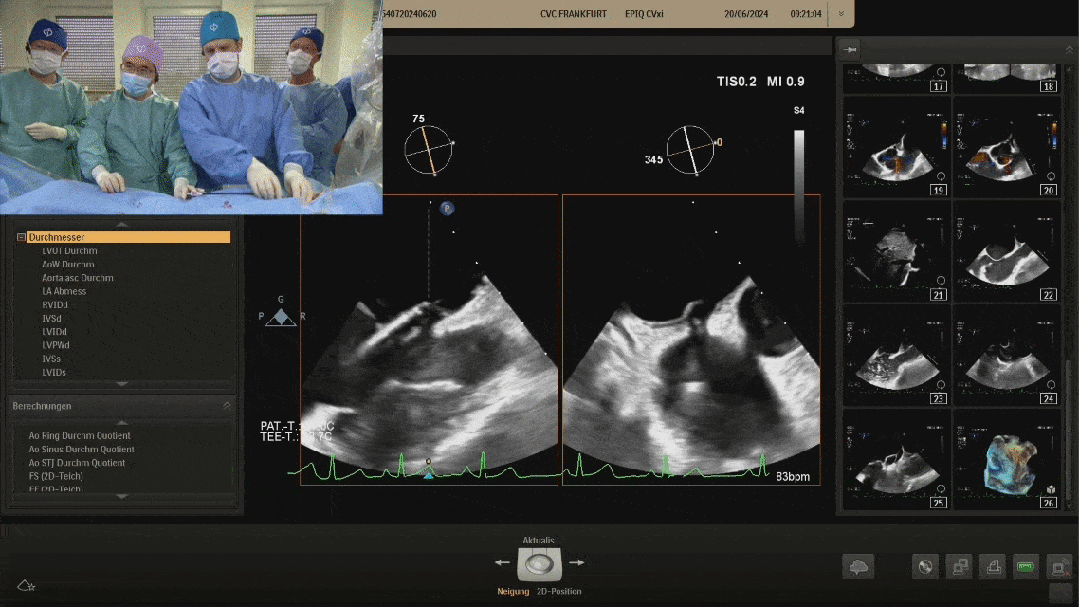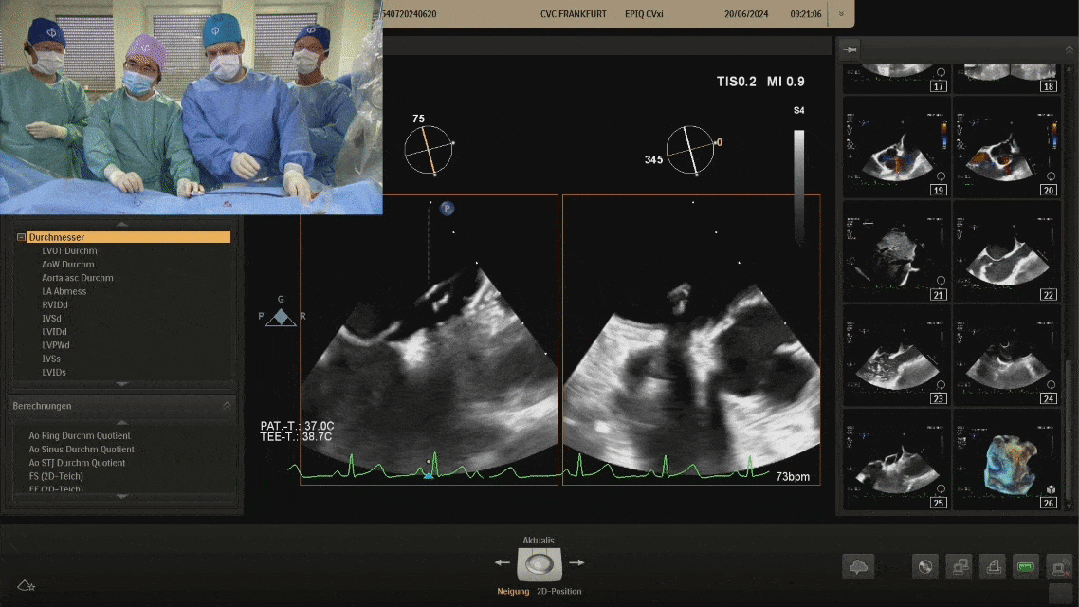
Confirm under ultrasound that both discs are tightly adhered to both sides of the septum, confirming the appropriate shape, and prepare to lock
Shaping and locking: Push the sheath forward, pull the shaping wire
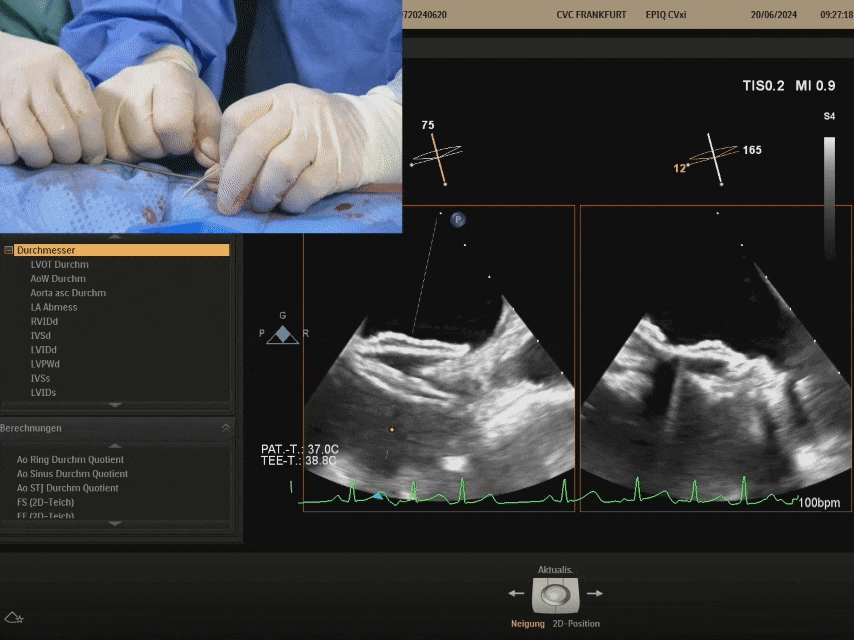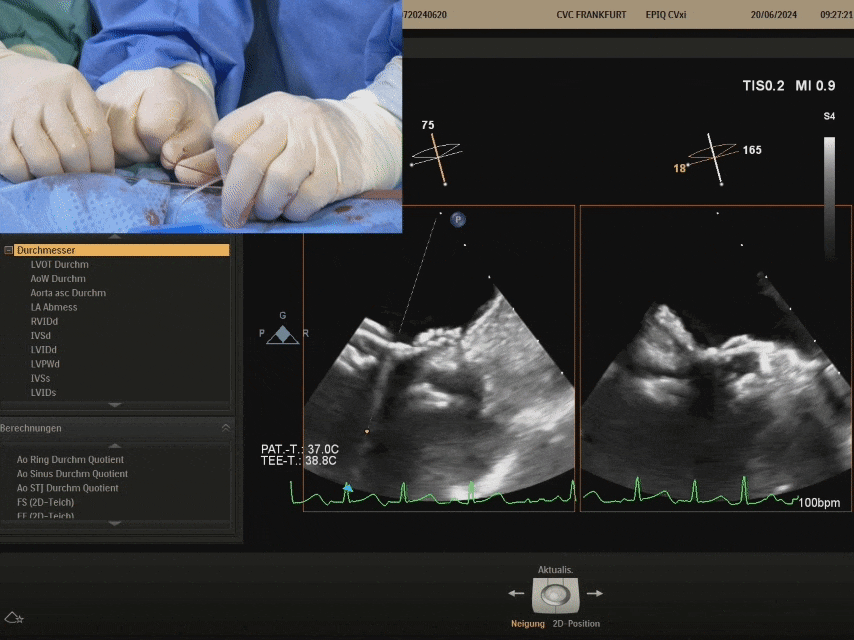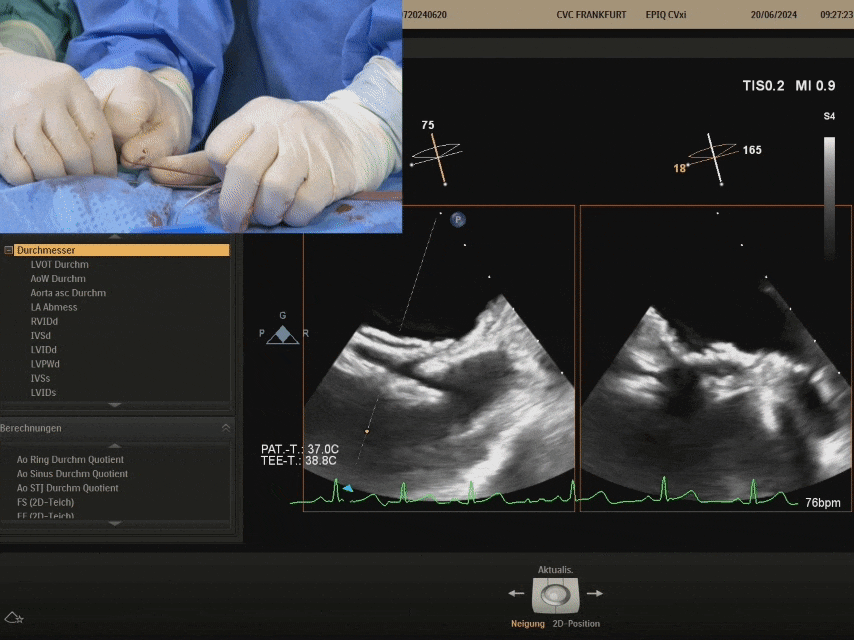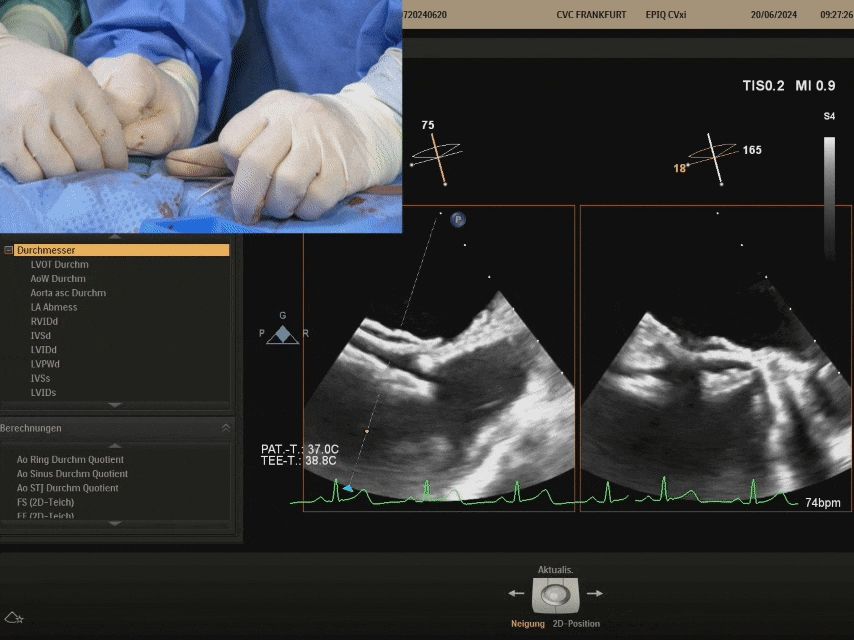
After shaping and locking, under ultrasound section, both discs tightly adhere to both sides of the septum, with a good shape
After locking, pull test
Gently pull the steel cable, the relative positions of the two discs remain unchanged, still tightly adhered to the septum, judging the locking success
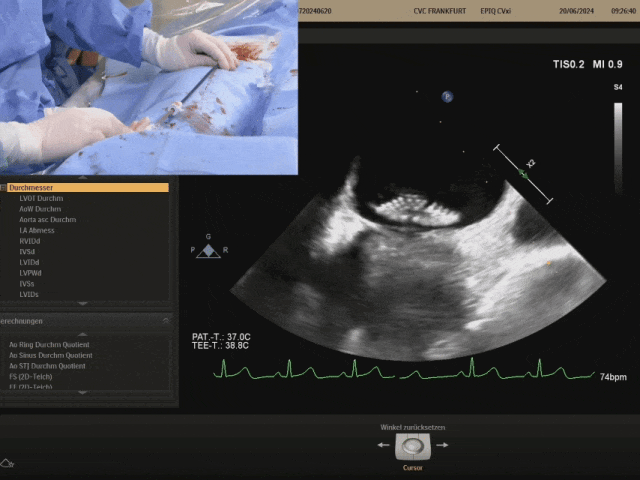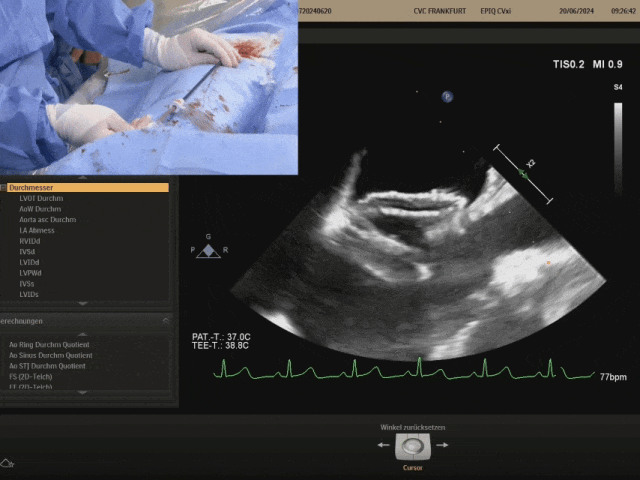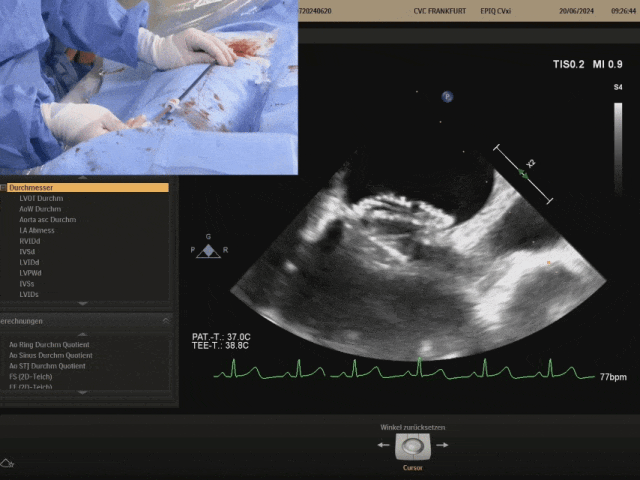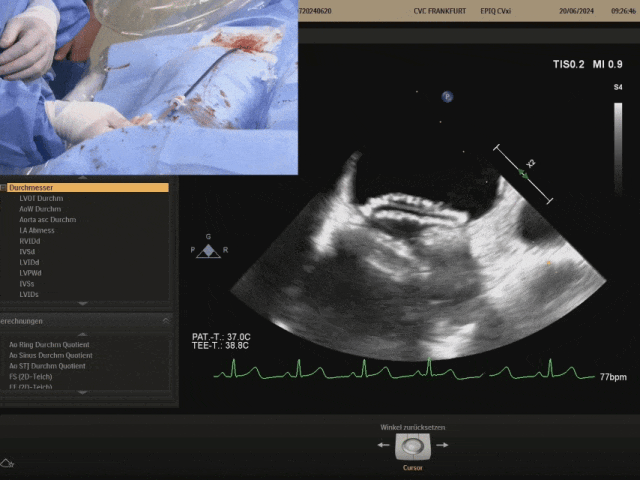
Cut the sutures and withdraw the shaping wire
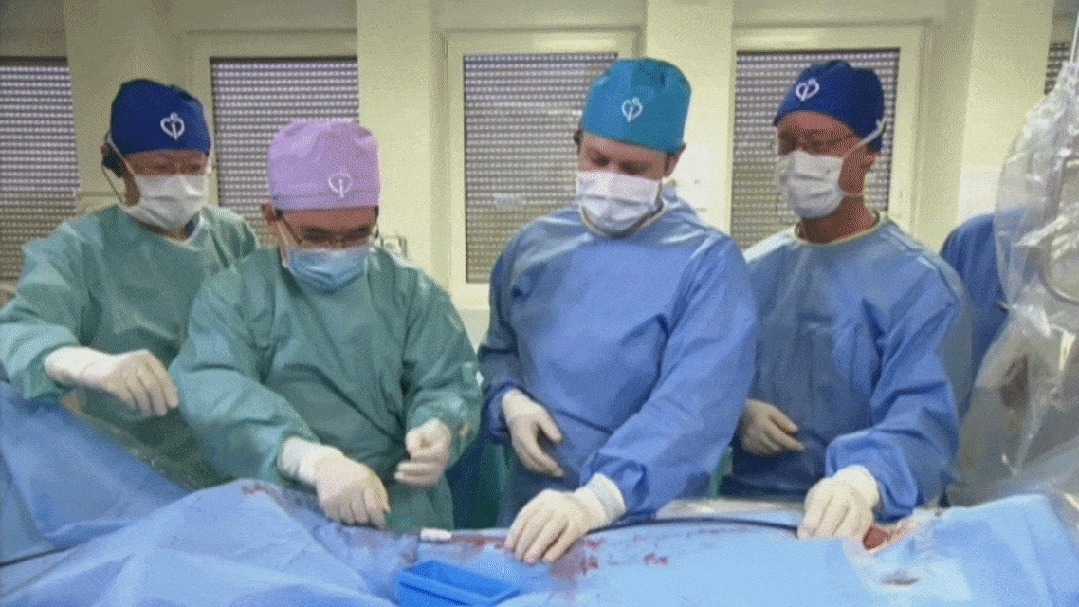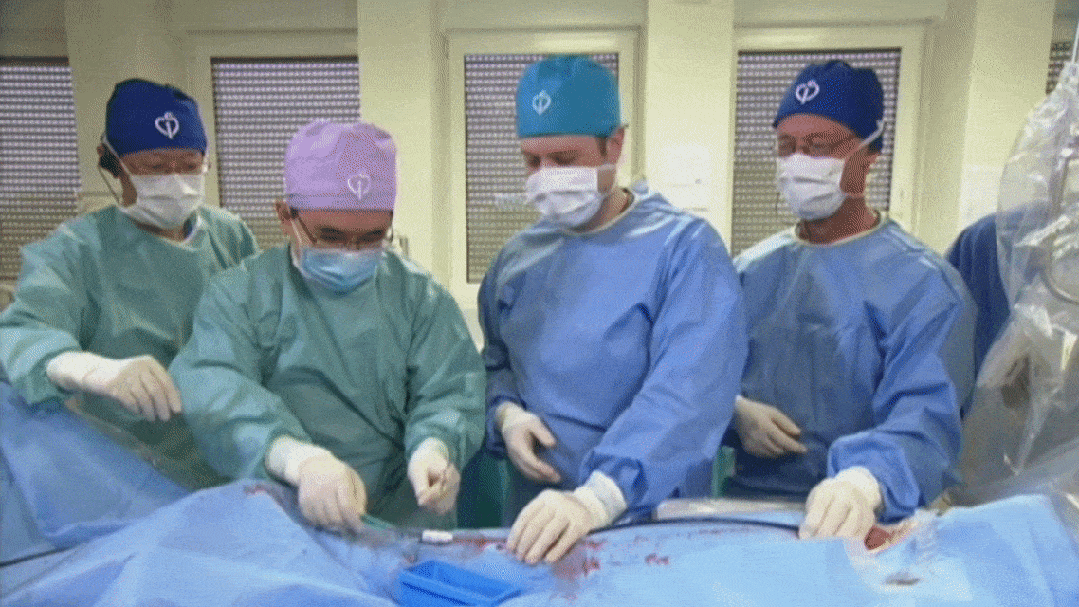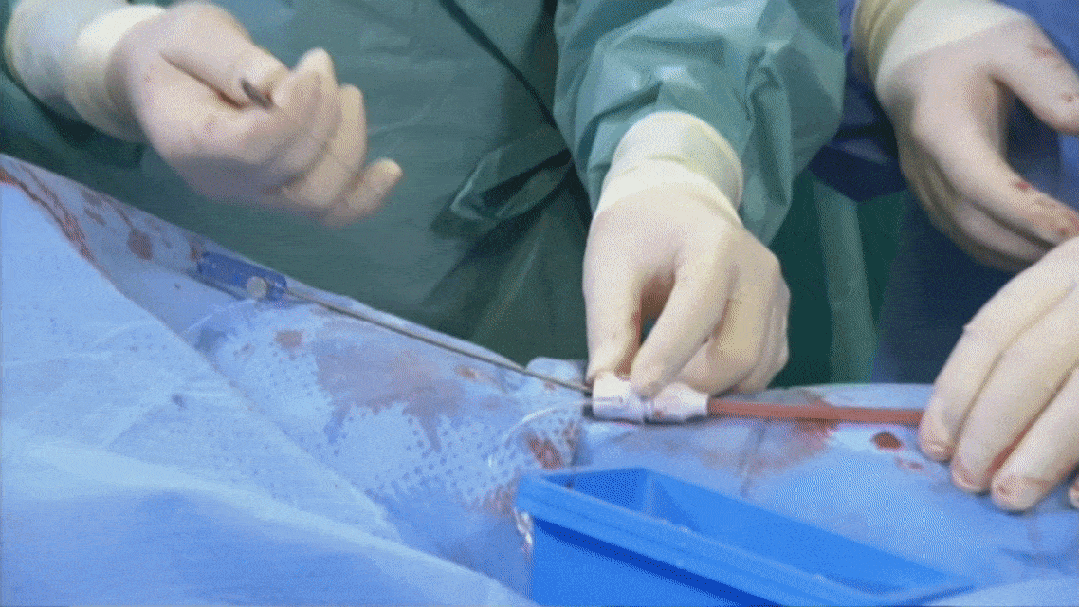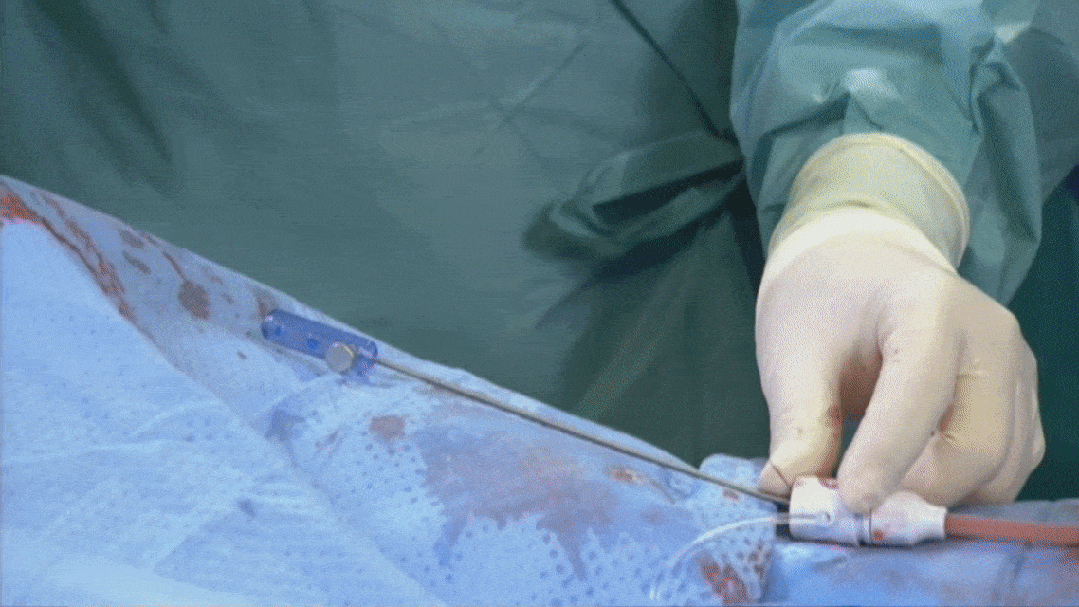
Suturing process
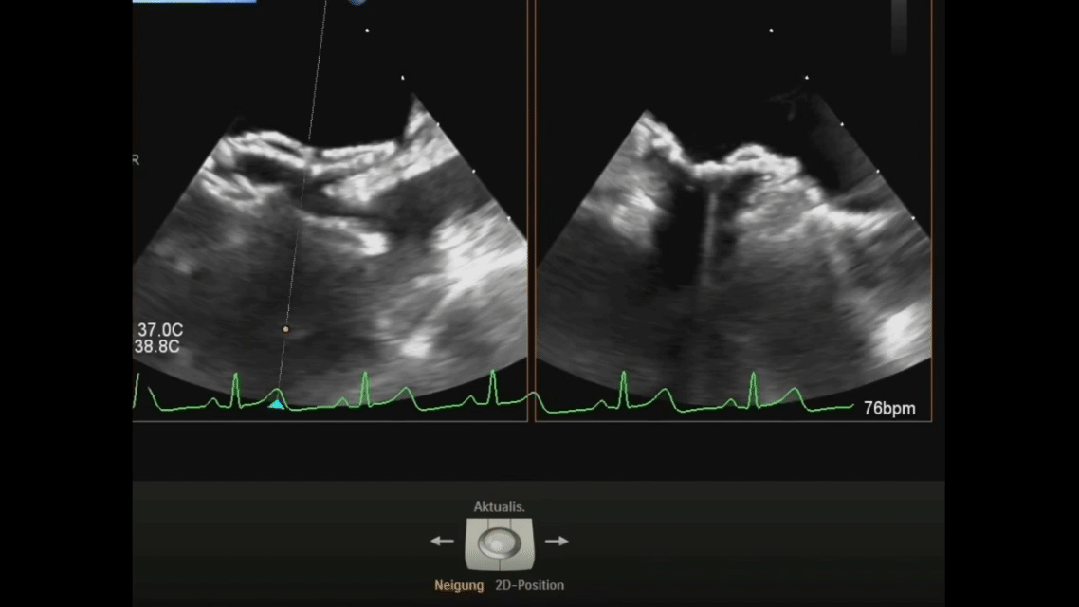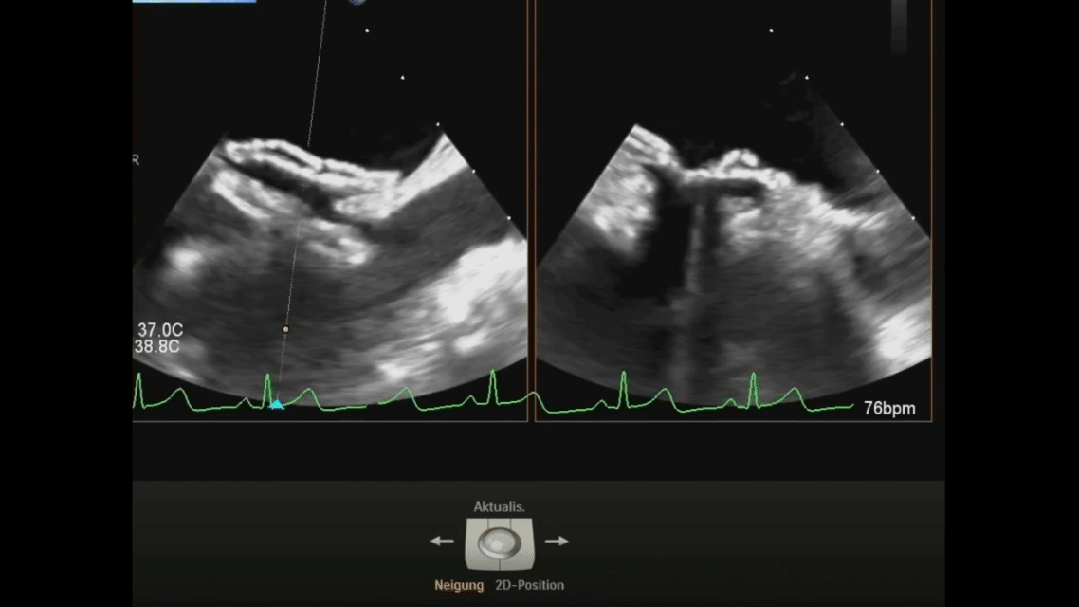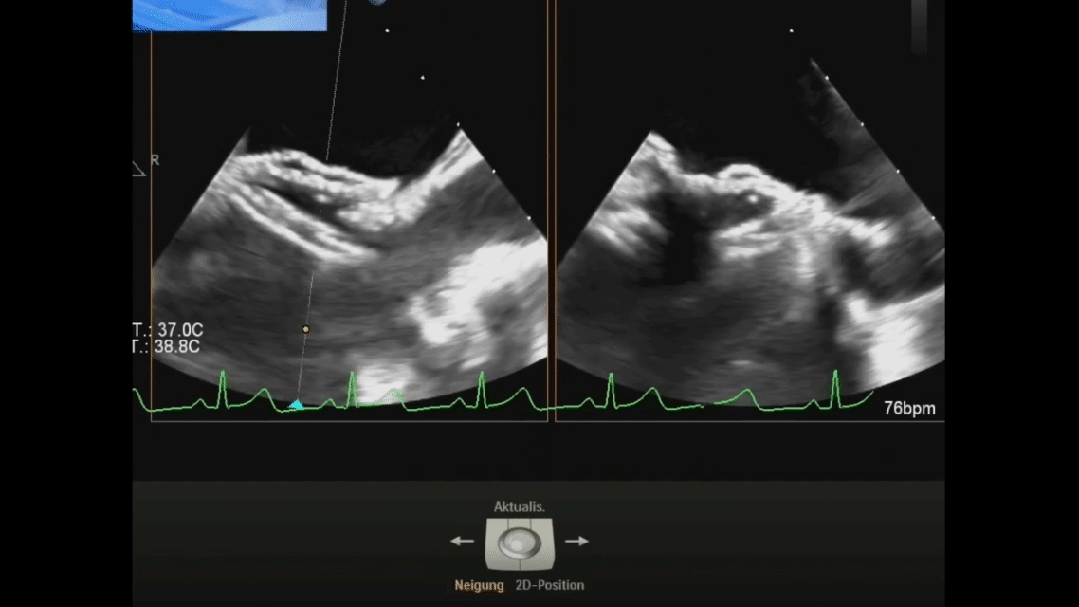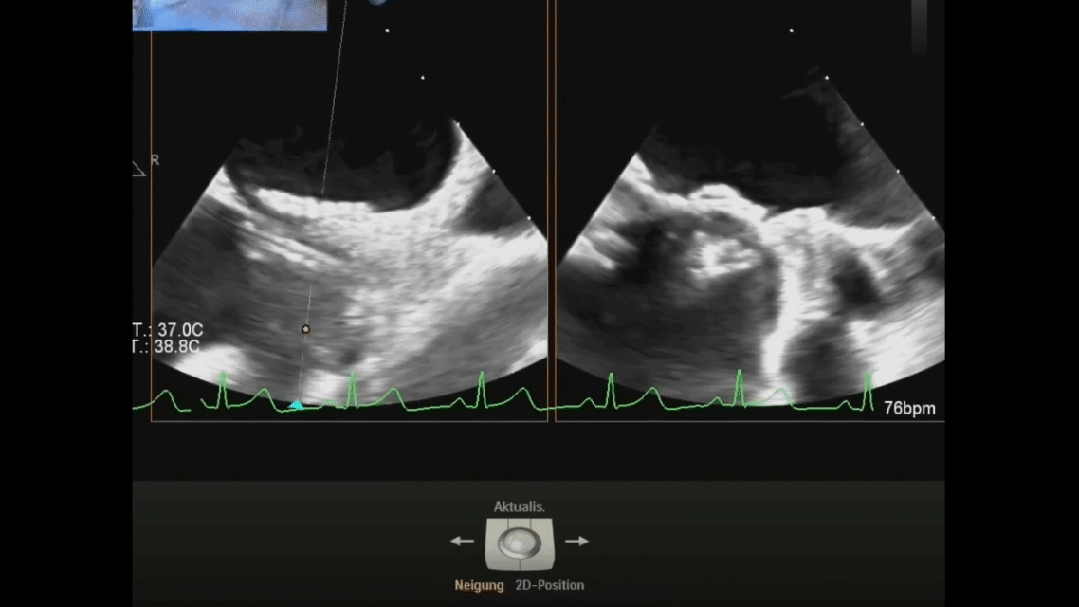
Ultrasound image during suturing process
Push the sheath forward to provide occluder support, rotate the steel cable counterclockwise. The relative positions of the two discs remain unchanged, the occluder shape is good, and the release is successful

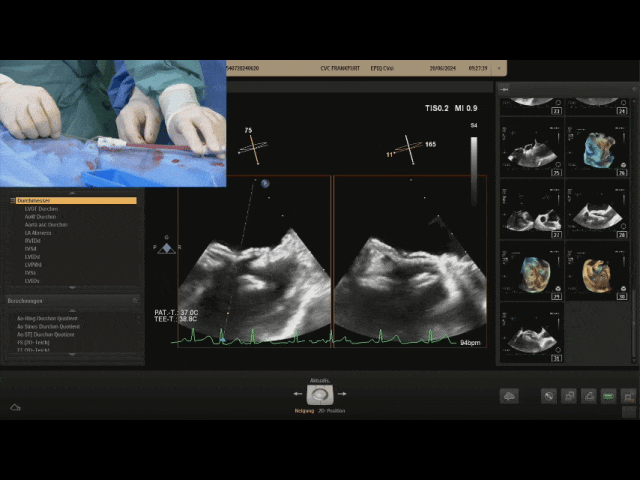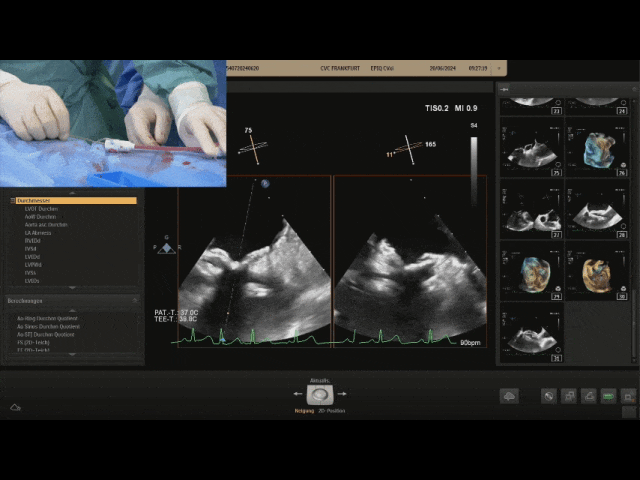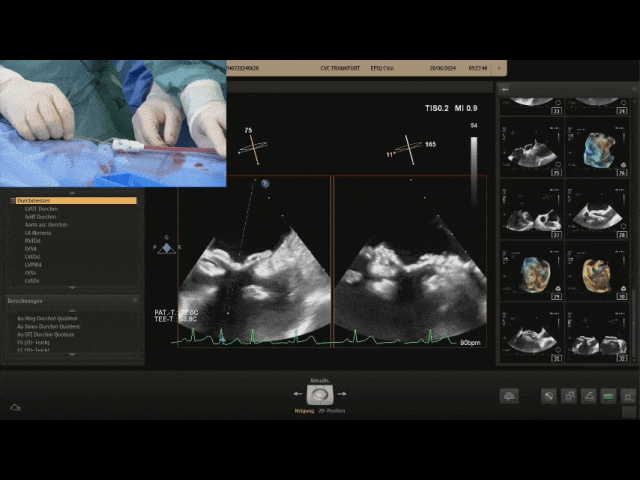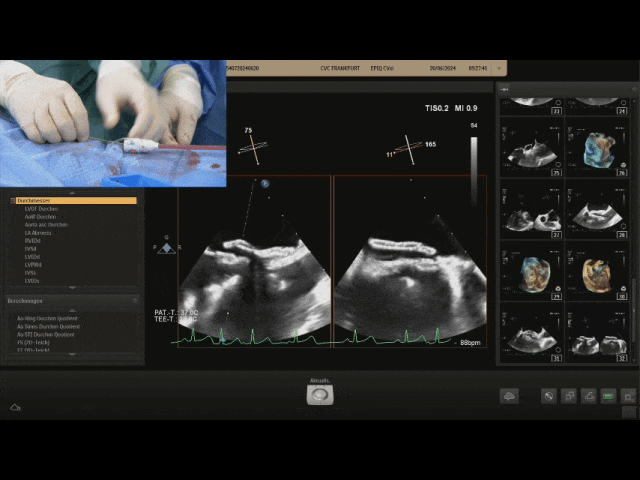
Withdraw the steel cable
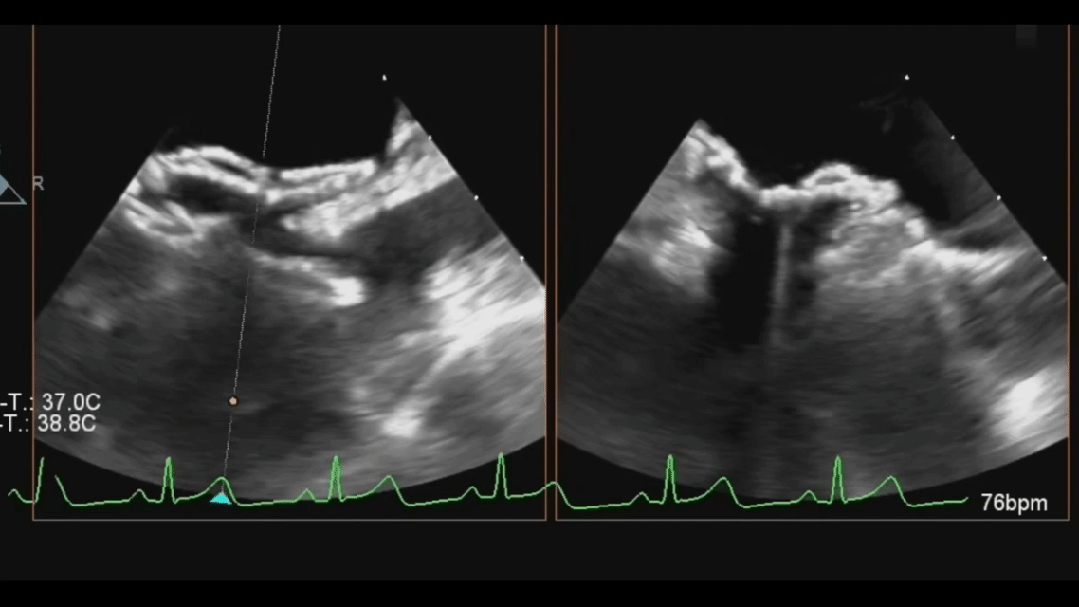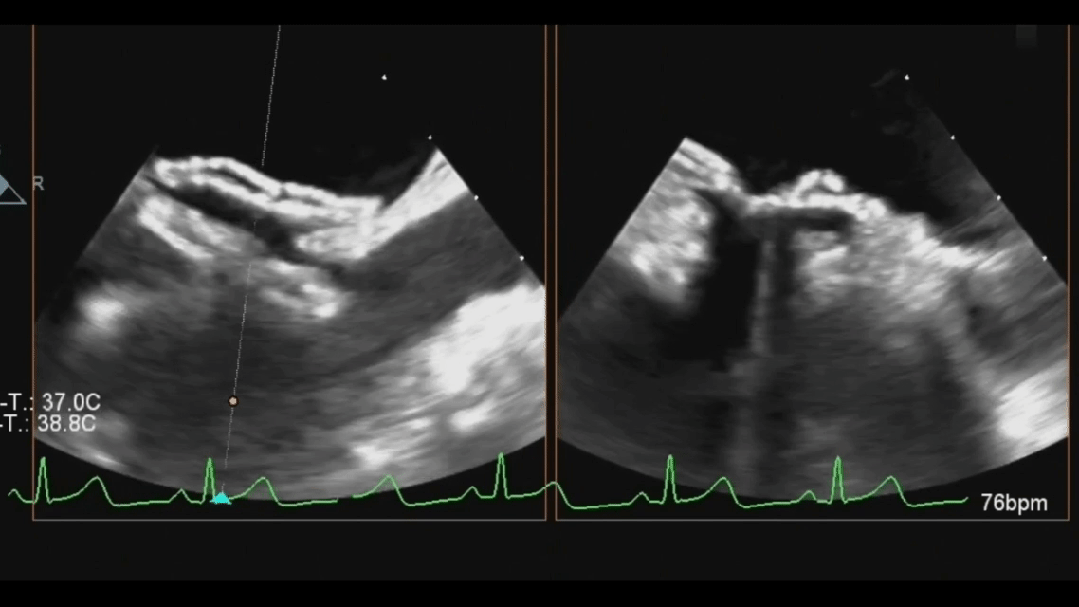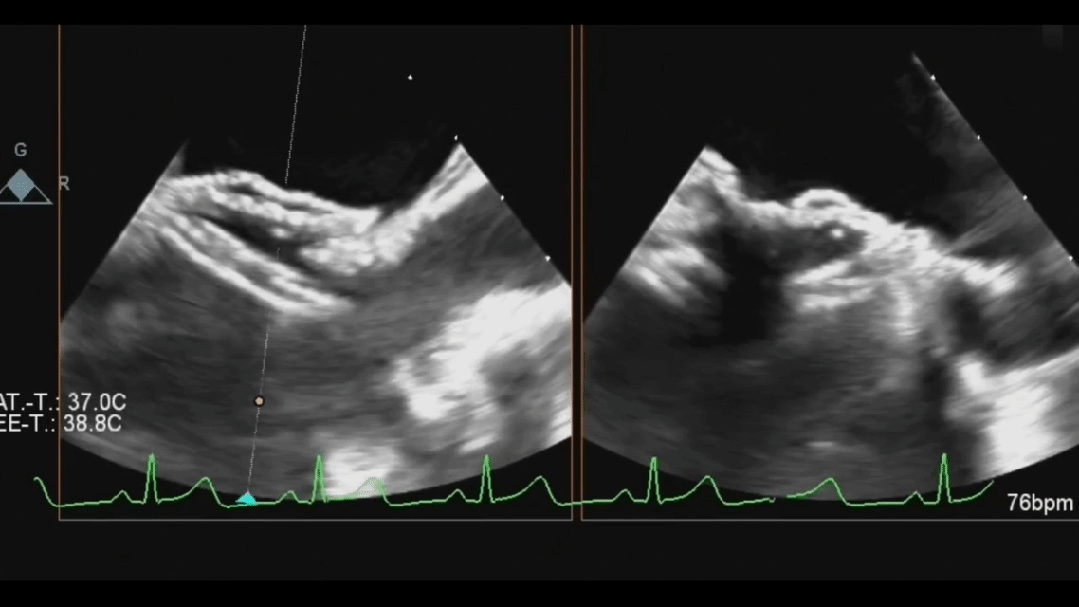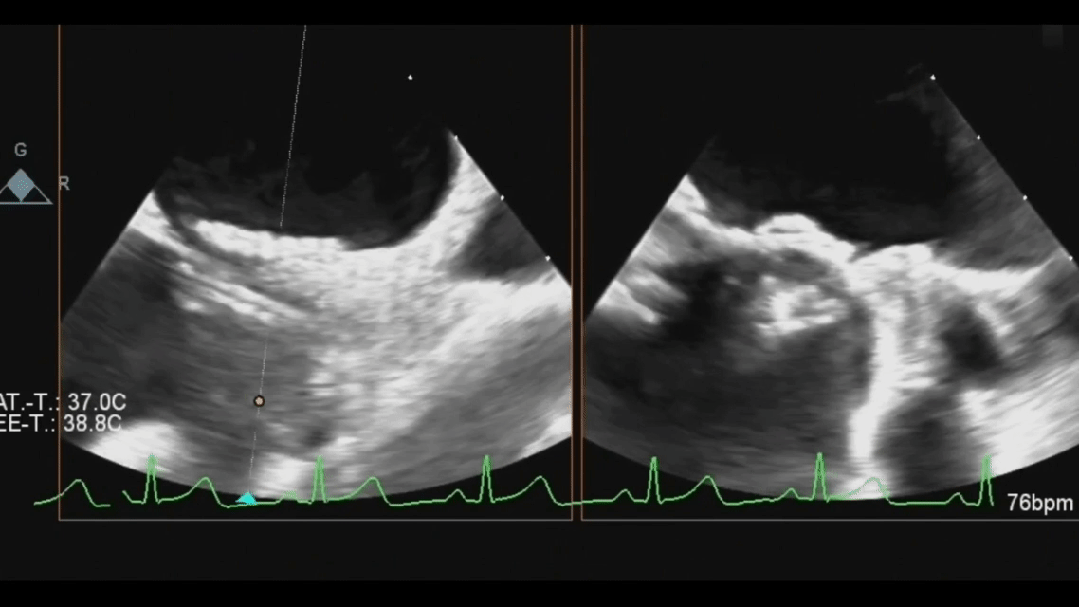
Ultrasound image during steel cable withdrawal process
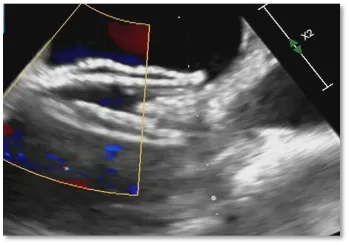
Postoperative ultrasound: The occluder shape is good, and the closure is successful
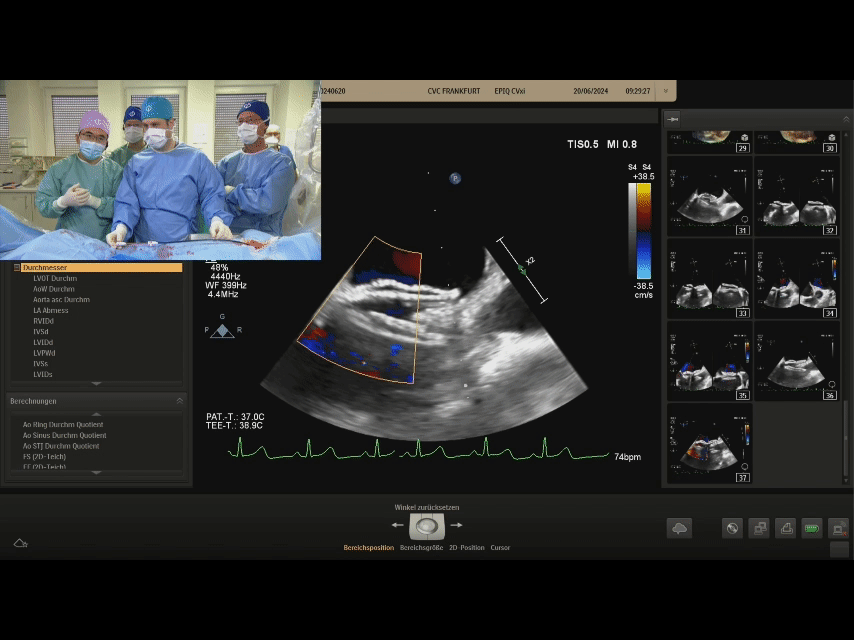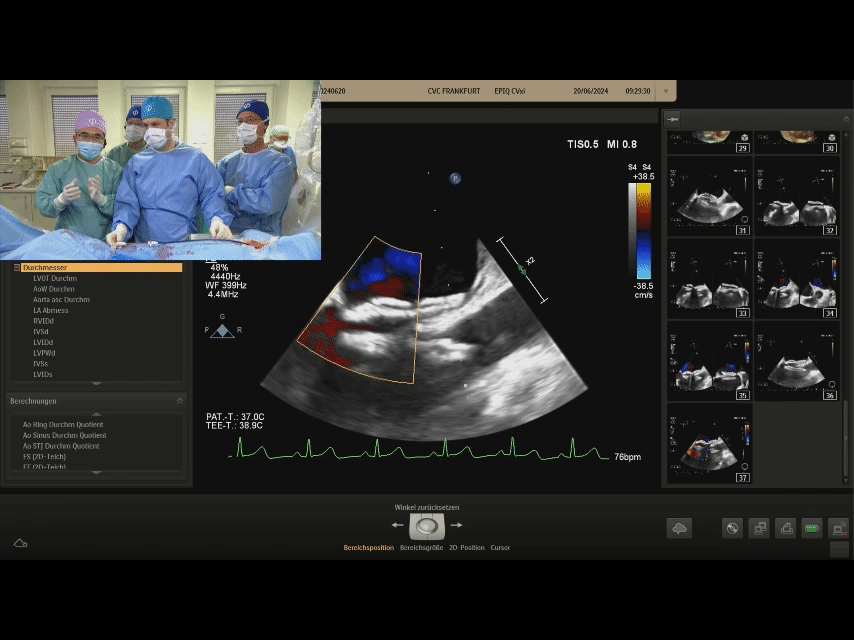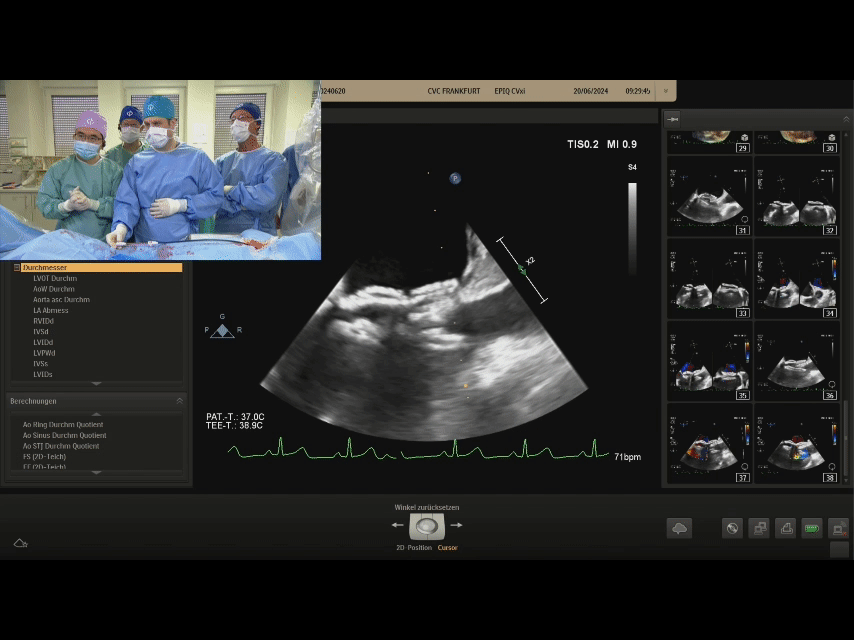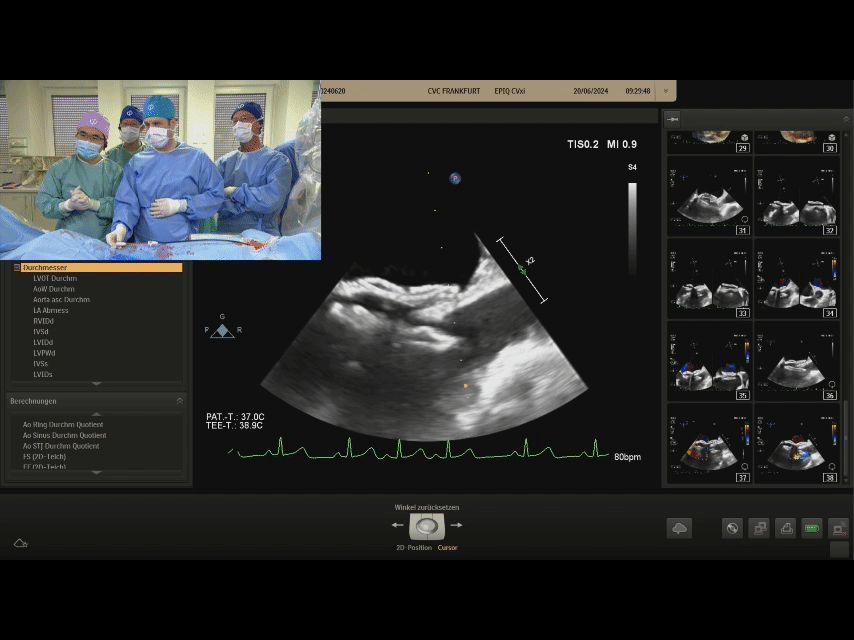

Case Summary
The biodegradable PFO occluder is composed of biopolymer materials. Under DSA (digital subtraction angiography), only the platinum ring of the occluder can be observed, necessitating ultrasound guidance and assessment during the occlusion procedure. Currently, there are three methods for ultrasound-guided PFO closure: transthoracic echocardiography (TTE), transesophageal echocardiography (TEE), and intracardiac echocardiography (ICE). For biodegradable occluders, combining ultrasound guidance ensures safe and precise implantation, reduces radiation exposure to patients and medical staff, decreases reliance on expensive equipment, and promotes environmentally friendly and cost-effective practices
The Pannawire intervention guidewire is specifically designed for pure ultrasound-guided procedures, with a shuttle-shaped tip that facilitates crossing PFOs. During the procedure, the Pannawire guidewire guides through the PFO tunnel to the left atrium, reducing the complexity of PFO crossing operation
The patient presented with a large, long-tunnel PFO measuring 14.4mm in length and 5.46mm in width. Choosing a biodegradable PFO occluder was considered advantageous as it conforms better to both sides of the septum, thereby preventing continued right-to-left shunting without impacting heart valve function. Hence, the BDPFO-I 3434 biodegradable PFO occluder was selected for closure during the procedure. Post-occlusion, ultrasound imaging confirmed the occluder's position and morphology were excellent, with complete closure and no residual shunting or pericardial effusion observed. There were no adverse reactions before or after closure, indicating a successful occlusion

发表留言
暂无留言
输入您的留言参与专家互动