多酚强化的糖萼样水凝胶涂层诱导心肌再生和免疫调节

HuiningWan,#XiaohuiYang,#YutongZhang,XiyuLiu,YanyanLi,YumeiQin,HuiYan,LanGui,KeLi,LongjianZhang,LiYang, Bo Zhang,* and Yunbing Wang
Although minimally invasive interventional occluders can effectively seal heart defect tissue, they still have some limitations, including poor endothelial healing, intense inflammatory response, and thrombosis formation. Herein, a polyphenol-reinforced medicine/peptide glycocalyx-like coating was prepared on cardiac occluders. A coating consisting of carboxylated chitosan, epigallocatechin-3-gallate (EGCG), tanshinone IIA sulfonic sodium (TSS), and hyaluronic acid grafted with 3-aminophenylboronic acid was prepared. Subsequently, the mercaptopropionic acid-GGGGG-Arg-Glu-Asp-Val peptide was grafted by the thiol−ene “click” reaction. The coating showed good hydrophilicity and free radical-scavenging ability and could release EGCG−TSS. The results of biological experiments suggested that the coating could reduce thrombosis by promoting endothelialization, and promote myocardial repair by regulating the inflammatory response. The functions of regulating cardiomyocyte apoptosis and metabolism were confirmed, and the inflammatory regulatory functions of the coating were mainly dependent on the NF-kappa B and TNF signaling pathway.
KEYWORDS:polyphenol-reinforced, hydrogel coating, cardiac occluders, myocardial regeneration, immunomodulation
Congenital heart disease refers to the abnormality of the heart caused by abnormal embryonic heart development, (1) and it is a major cause of neonatal morbidity and mortality. (2−4) Minimally invasive interventional cardiac occluders offer a number of advantages, such as less trauma, simple operation, and postoperative adjustment, (5−7) which have gradually become a mainstream treatment method. However, interventional occlusion also has many problems after implantation, such as endothelial injury, coagulation risk, and severe inflammatory response. (8−10) These problems limit the in vivo restorative effect of occluders, and it is important to upgrade the occluders to enhance their performance.
Various strategies have been considered to improve these performances and the researchers widely recognized that surface coating technologies can improve the performance of cardiovascular implants. Ding et al. (11) prepared a nanoscale 2D coating for left atrial appendage occluders using the filtered cathodic arc technique. This nanocoating consisted of a mixed titanium and substrate elements layer, a pure titanium layer, and an alternating layer of titanium and titanium nitride. The metal/ceramic alternative lamellar arrangement improved the adhesion of the coating to the substrate. This nanocoating had good material stability and blood compatibility and promoted the migration of endothelial cells (ECs) and rapid endothelialization of medical devices. Chen et al. (12) prepared a nitric oxide-eluting hydrogel coating consisting primarily of alginate and gelatin for use in vascular stents. By conjugating organoselenium species to alginate, the hydrogel persistently catalyzed the production of nitric oxide. The hydrogel coating inhibited thrombosis and inflammation and promoted rapid endothelial regeneration and was considered to modify many types of vascular implants. Kong et al. (13) designed a bioactive molecular dressing consisting of gelatin and laminin-derived A5G81 peptides for the polydioxanone (PDO) occluders. This bioactive modification can inhibit inflammation and excessive fibrosis and promote in situ tissue regeneration.
Recently, many studies, including those from our group, (14,15) have shown that epigallocatechin-3-gallate (EGCG) can regulate inflammation, ameliorate oxidative injury to cardiac cells, and reduce myocardial apoptosis. (16−19) Cai et al. (17) used EGCG and the rhein-peptide hydrogel (Rh-gel) coassembled to prepare an injectable EGCG@Rh-gel hydrogel system, which could achieve long-term continuous treatment, block the reactive oxygen species-inflammation cycle, and induce cardiomyocytes antiapoptosis to improve heart function. Tanshinone IIA sulfonic sodium (TSS), a water-soluble derivative of tanshinone IIA, (20,21) has been widely studied for its benefits, such as antiplatelet aggregation and anti-inflammatory activity. (20,22) Meanwhile, TSS can suppress cardiomyocyte hypertrophy and cardiac fibrosis, (23,24) and protect cardiomyocytes against oxidative stress-mediated apoptosis, (25,26) thus achieving cardioprotective effects.
The Arg-Glu-Asp-Val (REDV) is a tetrapeptide sequence, which selectively interacts with integrin α4β1 to capture REDV-specific cells, especially ECs, and promote their adhesion. (27−29) Zhang et al. (30) designed two triblock functional proteins consisting of adhesive domain, antifouling domain, and ECs-specific affinity domain (YIGSR and REDV peptide, respectively). The coating prepared with these triblock proteins exhibited a good biocompatibility and could selectively promote the adhesion and migration of ECs. Yang et al. (31) coated REDV-functionalized polyurethanes on the surfaces of cardiovascular implants via a “graft-to” method. The coatings reduced platelet adhesion and activation and showed selective attachment of ECs. Zhang et al. (28) prepared REDV/carboxybetaine (CB) coatings by conjugating the REDV peptide to a zwitterionic CB hydrogel, which allowed the surface to inhibit protein adsorption, platelet aggregation and activation, specifically capture ECs. Therefore, we considered that the REDV peptide could endow the material with the ability to capture ECs and had the potential for rapid re-endothelialization.
Based on the above considerations, we prepared a polyphenol-reinforced glycocalyx-like hydrogel coating for modifying cardiac occluders. The multifunctional glycans layer covering cells is called a glycocalyx, consisting mainly of glycoproteins and proteoglycans, and its biological functions have been studied in several aspects, including cardiovascular disease. (32) Hyaluronic acid (HA) is a glycosaminoglycan of the extracellular matrix and is present in the endothelial glycocalyx, (33,34) which is thought to provide a similar extracellular environment. (35,36) In our previous work, carboxylated chitosan (CCS), EGCG–TSS, and HA modified by 3-aminophenylboronic acid (APBA) were self-assembled on material surfaces to prepare a sandwich coating (37) by layer-by-layer self-assembly technology. Based on the advantages of the above sandwich self-assembled coating and REDV, we immobilized mercaptopropionic acid-GGGGG-REDV (SH-REDV) onto this sandwich coating via a thiol–ene “click” reaction to obtain the active peptide coating (CCS/EGCG–TSS/HA–APBA@REDV, CETHR) (Figure 1A), which was envisioned to provide a strategy to reduce thrombosis and achieve myocardial repair by adjusting the microenvironment steady state after occluders implantation. The composition, surface morphology, hydrophilicity, and potential of the coatings were investigated, and their free radical-scavenging ability, drug release ability, and in vitro biocompatibility of the coatings were evaluated. Meanwhile, the anti-inflammatory and proendothelialization abilities of CETHR and the potential gene level effect of the coating on H9c2 cells were probed.
Figure 1
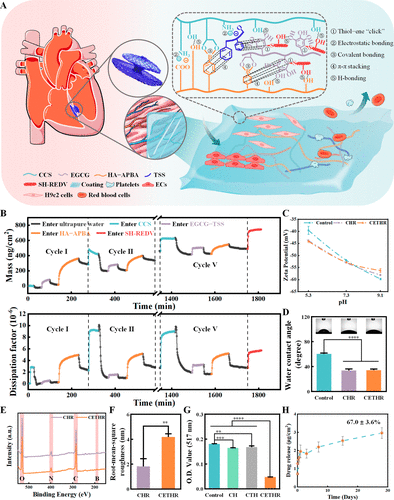
Figure 1. (A) Schematic diagram of the CETHR coating. (B) QCM-D experiment for real-time monitoring of the CETHR assembly process. Changes in M and D. (C) Zeta potential, (D) hydrophilic, (E) wide-scan XPS, and (F) root-mean-square roughness of different sample surfaces. (G) Free radical-scavenging capacity of different samples measured by DPPH assay. (H) Release of EGCG–TSS in the CETHR coating.
The dynamic assembly process was monitored by a quartz-crystal microbalance with dissipation (QCM-D) (Figure 1B). During the 5 cycles, the mass (M) of the adsorption layer on the surface gradually increased, and the variation of M and dissipation (D) value was ΔM = 489.0 ng/cm2 and ΔD = 2.7 × 10–6, respectively. The variation of the M of the adsorption layer gradually decreased during the cycles, which we considered to be due to the gradual decrease of the assembly driving force and the equilibration of the electric charge during the assembly process. Finally, after entering SH-REDV, the adsorption layers M and D changed with ΔM(SH-REDV) = 256.8 ng/cm2 and ΔD(SH-REDV) = 3.0 × 10–6, respectively. In X-ray photoelectron spectroscopy (XPS), clear signals of C, N, O, and B were detected on both CCS/HA–APBA@REDV (CHR) and CETHR surfaces (Figure 1E). The UV absorption peaks of HA–APBA at around 242 and 280 nm (Figure S1) were ascribed to the APBA. In addition, the atomic force microscopy (AFM) results showed that (Figures 1F and S2) the roughness of CHR and CETHR surfaces were about 1.8 ± 0.6 and 4.2 ± 0.3 nm, respectively. The above results suggested the successful preparation of CHR and CETHR coatings. The SH-REDV could be immobilized by thiol–ene “click” reaction, (38,39) and each component in the film interacted to form a stable coating.
Surface modification of materials leads to changes in their surface charges and surface hydrophilicity. As shown in Figure 1C, the zeta potential experiments showed negative surface potentials for the coating samples, with −43.8 ± 0.2 mV and −44.2 ± 0.3 mV for the CHR and CETHR coatings at pH about 5.3. As the pH improved, the surfaces presented stronger negative electrical and better electrical ionization level. Compared to the bare PDO surface (60.1 ± 1.6°), the modified surfaces (Figure 1D) showed better hydrophilicity (CHR for 33.5 ± 2.6° and CETHR for 34.0 ± 2.0°, respectively).
The 1,1-diphenyl-2-picrylhydrazyl (DPPH) assay indicated (Figures 1G and S3) that the CETHR coating had a good free radical-scavenging ability, considering that EGCG is a potent antioxidant and can eliminate oxidative stress. In addition, CETHR continuously released EGCG–TSS with a cumulative concentration of 3.0 ± 0.3 μg/cm2 for 28 days (Figures 1H and S4).
The adhesion of platelet-rich plasma (PRP) and whole blood on the sample surfaces were performed (Figure 2A,B). Compared with the control surfaces (93.0 ± 2.3 × 103 cells/mm2), the density of platelets was reduced on CHR (42.4 ± 16.8 × 103 cells/mm2) and CETHR (32.2 ± 12.8 × 103 cells/mm2). As shown in Figure 2D, the whole blood adhesion experiments showed similar results (7.2 ± 1.4 × 103 cells/mm2 for CETHR and 13.2 ± 1.6 × 103 cells/mm2 for unmodified surface). In addition, the hemolysis results (Figure S5) showed that the hemolysis rate of all groups was less than 5%, and the CHR and CETHR groups had a lower hemolysis rate than the control. Compared with the control (Figure S6), fewer blood components adhered to the surfaces of the CETHR group, which indicated that the CETHR coating had good antiblood adhesion after being rinsed with normal saline for 48 h.
Figure 2
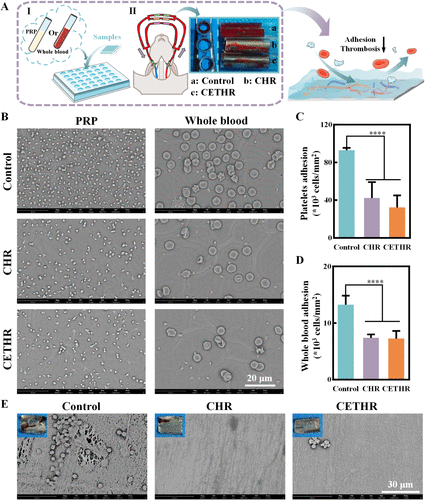
Figure 2. (A) Schematic diagram of PRP adhesion, whole blood adhesion, and ex vivo arteriovenous shunt test. (B) SEM images of adhered PRP and whole blood. Quantitative analysis of (C) platelets and (D) whole blood on different sample surfaces. (E) SEM images of the luminal surfaces of the samples in the ex vivo arteriovenous shunt assay. The blue background images in the top left corner are the images of the fixed samples after circulating blood for 1 h.
An ex vivo arteriovenous shunt assay (Figure 2A) was used to further evaluate antithrombotic ability. After blood circulation for 1 h, severe thrombosis was observed on unmodified surfaces consisting of platelets, erythrocytes, and cross-linked fibrin networks (Figure 2E). In contrast, thrombosis and occlusion were reduced on the CHR and CETHR surfaces. These results suggested that the CHETR had a good antithrombotic ability.
ECs and H9c2 cells were inoculated on the sample surfaces for culture (Figure 3A). The effect of the coatings on the ECs was evaluated, as shown in Figure 3B,C, the activity of ECs adhering to the CETHR coating was higher than that of the control group, and the coating contributed to the ECs adhesion. As shown in Figures 3D and S7, the migration distance of the ECs on the CETHR coating surface (143.7 ± 48.3 μm) was longer than that of the control (91.4 ± 18.0 μm). These results suggested that CETHR promoted the ECs adhesion and migration.
Figure 3
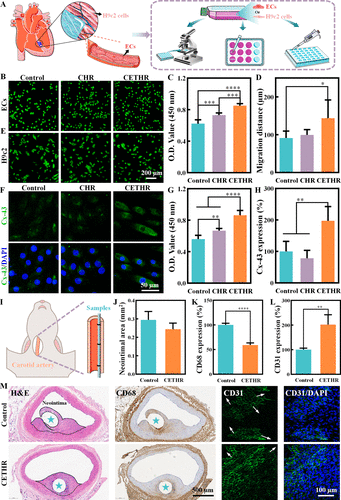
Figure 3. (A) Schematic diagram of the cell experiments. (B) FDA staining, (C) cell viability determined by the CCK-8 assay and (D) migration distance of ECs cultured on the sample surfaces. (E) FDA staining, (F) anti-Cx-43 antibody staining, (G) cell viability determined by the CCK-8 assay, and (H) Cx-43 expression level of H9c2 cells cultured on the sample surfaces. (I) Schematic diagram of in vivo carotid artery implantation experiments. Quantitative analysis of (J) neointimal area, (K) CD68 expression, and (L) CD31 expression on the neointima. (M) H&E, CD68, and CD31 staining of the carotid artery wrapped with implanted samples after implantation for 6 weeks. The sample locations are marked with asterisks.
We further evaluated the effect of the coatings on the H9c2 cells. The FDA staining and CCK-8 assay results (Figure 3E,G) showed that the coatings could promote the adhesion of H9c2 cells, and the highest activity of H9c2 cells was showed on the CETHR surface after 24 h. Similarly, the cellular Adenosine Triphosphate (ATP) activity assay results (Figure S8) showed that the CETHR group had higher intracellular ATP levels and cell activity compared to those of the control group.
Connexin-43 (Cx-43) is a functional protein that passes the electrical signal transmission among myocardial cells and promotes myocardial contraction, and plays an important role in maintaining the normal function of myocardium. (40−42) When the myocardium is damaged, the expression of Cx-43 protein is downregulated. The Cx-43 expression level of H9c2 cells was evaluated, and the Cx-43 expression level of H9c2 cells in the control was set to 100%. As shown in Figure 3F, dense Cx-43 protein was clearly observed in H9c2 cells in CETHR. Quantitative analysis (Figure 3H) showed that the expression level of Cx-43 protein in the CETHR group (197.4 ± 45.0%) was higher than the control group. In conclusion, the CCK-8, ATP activity, and Cx-43 staining assay results indicated that CETHR could protect H9c2 cells and promote adhesion.
The effects of the coatings on anti-inflammation and endothelialization were studied in a New Zealand white rabbit carotid artery model. Vessels and implanted samples were collected after 6 weeks; the outer circle was the natural vessel, and the inner circle was the neointima (Figures 3I and S9). Vessels were stained with hematoxylin and eosin (H&E) and anti-CD68. The neointimal area (Figure 3J,M) was 0.29 ± 0.05 mm2 for the control and 0.24 ± 0.03 mm2 for the CETHR group. The expression level of CD68 or CD31 in the control group was set to 100%. CD68 could be used to evaluate the degree of inflammation in the samples. Compared with the control group (Figure 3K,M), the CETHR coating had a lower degree of inflammatory cell infiltration and CD68 expression. In addition, the neointima was stained with anti-CD31 antibody to identify ECs, (14,43) and the staining results (Figure 3L,M) showed that the endothelial layer surrounding the neointima tissue was more intact and the expression level of CD31 was stronger (201.8 ± 39.8%) in the CETHR group compared with the control. The above experimental results suggested that the CETHR coating could suppress inflammation and promote endothelialization.
Subcutaneous implantation into Sprague–Dawley (SD) rats was performed to further evaluate histocompatibility (Figure 4A).After 2 and 4 weeks, the tissues around the implants were taken out for H&E staining. As shown in Figure 4B,C, there were different degrees of fibrosis around all implants. The thickness of the fibrous capsule after 4 weeks for coating groups was lower than that for the control (176.6 ± 7.0 μm), with the thinnest fibrous capsule thickness for CETHR (60.1 ± 4.3 μm).
Figure 4
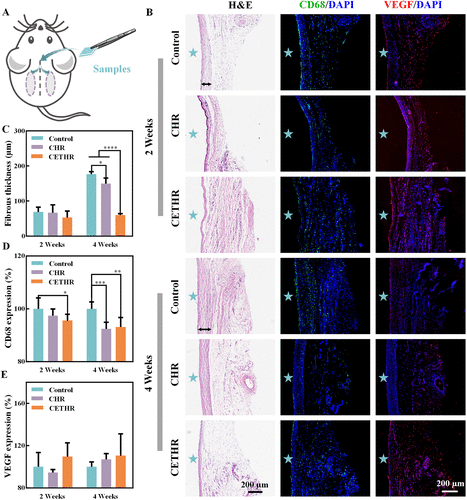
Figure 4. (A) Schematic diagram of subcutaneous implantation. (B) H&E, CD68/DAPI, and VEGF/DAPI staining of tissues after subcutaneously implanted samples for 2 and 4 weeks. The black line indicates the fibrous capsule, and the implanted sample locations are marked with asterisks. Quantitative analysis of (C) the thickness of fibrous capsule and (D) CD68 and (E) VEGF expression in tissues surrounding subcutaneously implanted samples.
In addition, anti-CD68 antibody and anti-vascular endothelial growth factor (VEGF) antibody immunofluorescence staining was performed. VEGF promoted angiogenesis and regulated endothelial function. (44−46) CD68 and VEGF expressions levels of the control group were both set to 100%. As shown in Figure 4B,E, there was no statistically significant difference in VEGF expression levels among different groups. In contrast, the CD68 staining results (Figure 4B,D) showed that the expression levels of CD68 in the CETHR group were 95.6 ± 2.4% and 93.2 ± 3.6% after 2 and 4 weeks, respectively, which indicated that the inflammatory cell infiltration was lower for the CETHR group. In short, the above results showed that the CETHR coating inhibited the inflammatory reaction and exhibited good histocompatibility.
We further investigated the effect of CHETR coating on the gene level in H9c2 cells by RNA-sequencing analysis. A total of 11607 genes were detected. Under the conditions of an absolute value of fold change (FC) greater than 1.2 and a p value less than 0.05, the CETHR coating group downregulated 134 genes and upregulated 167 genes compared with the control group (Figure 5A), and the heat map of the differentially expressed genes under these conditions is shown in Figure 5D. The above differentially expressed genes were analyzed by the Kyoto Encyclopedia of Genes and Genomes (KEGG), which linked genomic information with different functional information (Figure 5B,C). The cellular apoptosis and metabolism associated pathways included the cAMP signaling pathway, cGMP-PKG signaling pathway, MAPK signaling pathway, and apoptosis. The cellular inflammation directly associated pathways included the NF-kappa B signaling pathway and the TNF signaling pathway. In addition, the gene interactions were analyzed using the STRING database (Figure 5E).
Figure 5
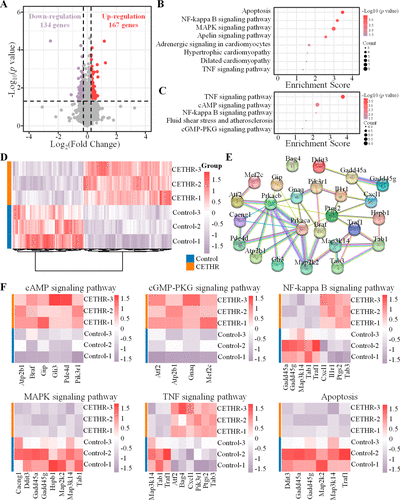
Figure 5. RNA-sequencing analysis of H9c2 cells on sample surfaces. (A) The volcano plot showing differentially expressed genes of H9c2 cells in control and CETHR groups. Red and purple represent up- and down-regulated genes, respectively. Bubble diagram of KEGG enrichment analysis of (B) downregulated and (C) upregulated gene pathways. (D) Heat map of differentially expressed genes in H9c2 cells with |FC| > 1.2 and p < 0.05. (E) Interaction network of genes through the STRING database. (F) Heat map of differentially expressed genes of related signaling pathways.
While mild inflammation usually subsides in time for the benefit of the host, an unbalanced inflammatory response can lead to lasting tissue damage. Nuclear factor kappa B (NF-κB), a key transcription factor, is related to many genes involved in immune and inflammatory processes and can induce the expression of proinflammatory genes. (47,48) NF-κB plays a key role in cardiomyocyte hypertrophy, and dysregulated NF-κB activation contributes to various inflammatory diseases and myocardial damage. (49−51) In addition, tumor necrosis factor (TNF)-α is an inflammatory cytokine that can activate NF-κB, and is associated with the transcription of various proinflammatory genes. (48,52) The analysis confirmed that compared with the control group (Figure 5E,F), the CETHR had the potential to regulate the inflammatory reaction of H9c2 cells by regulating the genes related to NF-κB and TNF (Gadd45a, Gadd45g, Map3k14, Tab1, Traf1, Cxcl1, Il1r1, Ptgs2, Tab3, Atf2, Bag4, and Pik3r1).
Apoptosis can lead to the death of many cardiomyocytes, which plays an important role in the progression of myocardial injury. (53) The CETHR coating downregulated the genes involved in apoptosis (Ddit3, Gadd45a, Gadd45g, Map2k2, Map3k14, Traf1) to inhibit myocardial injury. Meanwhile, the MAPK signaling pathway has been proven to be related to cell survival and apoptosis. (54,55) In the CETHR coating group, genes expressed in the MAPK signaling pathway (Cacng1, Ddit3, Gadd45a, Gadd45g, Hspb1, Map2k2, Map3k14, Tab1) were downregulated to inhibit H9c2 cells apoptosis. In addition, cAMP regulates several key physiological processes in the heart. The CETHR coating upregulated genes involved in the cAMP signaling pathway (Atp2b1, Braf, Gip, Gli3, Pde4d, Pik3r1), regulated Ca2+ homeostasis, and inhibited apoptosis and necrosis. (56,57) Similarly, in the cGMP-PKG signaling pathway, the up-regulated genes included Atf2, Atp2b1, Gnaq, and Mef2c, thus triggering cardioprotection. (58,59) The above results showed that the CETHR coating had the potential to reduce myocardial damage and played a role in cardioprotection and promotion of myocardial repair by regulating metabolism and cellular inflammation, and inhibiting apoptosis.
A polyphenol-reinforced medicine/peptide glycocalyx-like coating (CETHR) was prepared on cardiac occluders via layer-by-layer self-assembly technology and a thiol–ene “click” reaction. The coating could scavenge free radicals, promote ECs adhesion and migration, and protect H9c2 cells with good hemocompatibility and histocompatibility. In addition, the inflammatory regulatory functions of the CETHR were mainly dependent on the NF-kappa B and TNF signaling pathways, and the CETHR could regulate the apoptosis and metabolism of H9c2 cells mainly through the cAMP signaling pathway, cGMP-PKG signaling pathway, MAPK signaling pathway, and apoptosis. With these promising results, we considered that this functional coating for cardiac occluders maintained microenvironmental homeostasis, reduced thrombotic risk by accelerating the endothelialization process, and further regulated inflammation to promote myocardial tissue repair.
CCS, APBA, and eosin Y (water soluble) were purchased from Aladdin Reagent Co., Ltd. (Shanghai, China). HA (Mw ∼ 100,000) was obtained from Qufu Guanglong Biochemical Factory (Shandong, China). TSS was purchased from Shanghai yuanye Bio-Technology Co., Ltd. (Shanghai, China). EGCG was purchased from Wuxi Taiyo Green Power Co., Ltd. (Jiangsu, China). SH-REDV peptide was purchased from Hangzhou All Peptide Biotechnology Co., Ltd.(Hangzhou, China). DPPH free radical was purchased from TCI (Shanghai) Development Co., Ltd. The PDO filaments were obtained from Shanghai Shape Memory Alloy Ltd. (Shanghai, China). 3-Hydroxytyramine hydrochloride, 2-morpholinoethanesulfonic acid, 3-(3-dimethylaminopropyl)-1-ethylcarbodiimide hydrochloride, and N-hydroxysuccinimide were purchased from Adamas Reagent Co., Ltd. (Shanghai, China).
The CETHR coating was fabricated by layer-by-layer self-assembly technology (37) and thiol–ene “click” reaction. The bare substrates were selected as a control, and the preparation details of these coatings are described in Supporting Information M1.
The self-assembly process of the coating was monitored by QCM-D (Qsense Analyzer, Biolin scientific, Sweden) in real time, and the detailed method is described in Supporting Information M2. The surface chemical compositions were further studied by XPS (ESCALAB Xi+, Thermo Fisher, USA) with Al Kα radiation (hv = 1486.6 eV). The micromorphology and roughness of the coating were verified by AFM (Dimension ICON, Bruker, Germany). The hydrophilicity of the samples was measured by a WCA analyzer (Theta, Biolin, Sweden). In short, a certain amount of ultrapure water was dropped onto the surfaces of the samples at room temperature. SurPASS 3 instrument (Anton Paar, Austria) was used to verify the surface zeta potential of the samples, in which hydrochloric acid and potassium hydroxide were used to adjust the pH value. In the above experiments, PDO sheets were used as the bare substrates, except 316L stain steels sheets were used as the substrates for the AFM and XPS experiments, and gold-plated quartz crystals were used as the substrates for the QCM-D test.
For free radical-scavenging assays, the coatings were prepared on the PDO sheets. DPPH (0.1 mM) was dissolved in anhydrous ethanol. The bare or coating samples with 500 μL of DPPH solution were incubated for 30 min away from light at 37 °C, and 200 μL of the liquid was taken, and the absorbance was measured at 517 nm.
The CETHR coatings were prepared on PDO sheets and immersed in 4 mL of phosphate buffered saline (PBS, pH ∼ 7.4). Then, 1.2 mL of fluid was harvested, and 1.2 mL of fresh PBS was replenished at predetermined time points. The absorbance of the release solution was measured using a UV–visible spectrophotometer (Evolution 220, Thermo Fisher Scientific, USA) and the release of EGCG–TSS was determined from a standard curve (λ = 279 nm). The coating was disrupted by ultrasound to evaluate the loading of the EGCG–TSS.
For blood-contact materials, it is required that they have good blood compatibility. Fresh New Zealand white rabbit blood was obtained from the West China Experimental Animal Center of Sichuan University. All animal experiments followed the Guidelines for Care and Use of Laboratory Animals of Sichuan University and they met the ethical guidelines. We selected PDO sheets as the bare substrates and used PRP and whole blood adhesion to evaluate the blood compatibility of coating samples in vitro. PRP was obtained by centrifuging fresh rabbit blood at 340 × g. The samples were incubated with PRP or whole blood at 37 °C for 1 h, and then the sample surfaces were gently washed, fixed with 2.5% glutaraldehyde (4 °C), dehydrated, and observed by scanning electron microscopy (SEM, Phenom Pro, Thermo Fisher Scientific). In addition, the blood compatibility of the samples was further evaluated by a hemolysis test. The antithrombotic properties of the samples after normal saline rinsing were evaluated by a peristaltic pump rinsing test. The details of hemolysis test and peristaltic pump rinsing test are described in Supporting Information M3 and M4
The antithrombotic ability was evaluated by an ex vivo arteriovenous shunt assay. In this experiment, male New Zealand white rabbits were selected, and the coatings were prepared on 316L stain steels. Under anesthesia, the arteriovenous extracorporeal circuit was connected by cannulas through the left carotid artery and the right external jugular vein of the rabbits. The bare and coating samples were placed into the cannulas and the back sides of the samples were tightly attached to the inside surfaces of the cannulas. After monitoring the circulating blood flow for 1 h, the samples were gently cleaned and photographed. The samples were collected, fixed, dehydrated, and observed by SEM.
Promoting endothelialization of the device and repairing of the myocardial tissue are important goals of the occludes. In order to evaluate the influence of coating materials on cell behavior, ECs or H9c2 cells were inoculated on the sample surfaces for culture.
The coatings were prepared on PDO sheets. The bare and coating samples were placed in well plates and then added with cell culture medium containing ECs. After incubation for 24 h, the cells were observed by FDA staining, and the CCK-8 experiment was used to evaluate the ECs viability of the sample surfaces. The effect of the coatings on the migration behavior of ECs was further explored. The coatings were prepared on 12-well plates, and the digested ECs were inoculated directly on the surfaces of the bare or coated samples to form a confluent cell monolayer. The cell monolayers were scraped with a 1 mL pipette tip to form linear scratches and then incubated for 24 h after slight washing with PBS, in which ECs were cultured in serum-free medium.
Similarly, the coatings were prepared on the PDO sheets, and H9c2 cells were inoculated on the surfaces of the samples. After incubation for 24 h, FDA staining and CCK-8 experiment were performed. H9c2 cells cultured for 72 h were fixed with 4% paraformaldehyde and stained with anti-Cx-43 antibody to evaluate Cx-43 expression in H9c2 cells. In addition, the coatings were prepared directly on 6-well plates, and H9c2 cells were inoculated on the surfaces. After incubation for 36 h, intracellular ATP levels were detected using a Rat ATP ELISA Kit (Bioswamp).
The effects of coatings on inflammation and endothelialization were evaluated using the New Zealand white rabbit carotid artery model. The coatings were prepared on PDO filaments used to prepare occluders. The bare and coating samples were implanted and fixed onto the inner surfaces of the carotid arteries. After 6 weeks, the vessels containing the samples were collected and fixed. Then, they were stained with H&E, anti-CD68, and anti-CD31 antibodies.
The coatings were prepared on PDO sheets, and the bare and coating samples were subcutaneously implanted into SD rats to detect histocompatibility. The fibrous hyperplasia tissues were harvested after 2 and 4 weeks, and the tissues were stained with H&E, anti-CD68 antibody and anti-VEGF antibody. The details of subcutaneous implantation are described in Supporting Information M5.
We used RNA-sequencing analysis to identify the effect of CHETR coating on the gene level in H9c2 cells. For RNA-sequencing analysis, the coatings were prepared directly on 6-well plates, and H9c2 cells were inoculated on the bare or coating sample surfaces and incubated for the same time to the appropriate number. Subsequently, 0.8 mL of TRIzol Reagent (Ambion) was added and blown until the cell layer was completely dissolved and then stored at −80 °C for subsequent experiments.
An appropriate amount of total RNA was selected from samples to construct the RNA-sequencing library, which was inspected by an Agilent 2100 Bioanalyzer and quantified by the qPCR method. Sample library sequencing was carried out using an Illumina NovaSeq 6000. The library construction and sequencing services were provided by Aksomics (Shanghai).
The data results are expressed as mean ± standard deviation. t-Test, one-way ANOVA, or two-way ANOVA were used to evaluate statistical significance among the samples, and *p < 0.05 indicated a significant difference. The symbols **, ***, and ****represent p < 0.01, p < 0.001, and p < 0.0001, respectively.
Corresponding Author
Bo Zhang - National Engineering Research Center for Biomaterials, Sichuan University, Chengdu, Sichuan 610065, China; Bioengineering Department, University of California, Los Angeles, California 90095, United States; Orcidhttps://orcid.org/0000-0001-5902-0290; Email: bozhang@scu.edu.cn
Authors
Huining Wan - National Engineering Research Center for Biomaterials, Sichuan University, Chengdu, Sichuan 610065, China
Xiaohui Yang - National Engineering Research Center for Biomaterials, Sichuan University, Chengdu, Sichuan 610065, China
Yutong Zhang - National Engineering Research Center for Biomaterials, Sichuan University, Chengdu, Sichuan 610065, China
Xiyu Liu - National Engineering Research Center for Biomaterials, Sichuan University, Chengdu, Sichuan 610065, China
Yanyan Li - National Engineering Research Center for Biomaterials, Sichuan University, Chengdu, Sichuan 610065, China
Yumei Qin - National Engineering Research Center for Biomaterials, Sichuan University, Chengdu, Sichuan 610065, China
Hui Yan - National Engineering Research Center for Biomaterials, Sichuan University, Chengdu, Sichuan 610065, China
Lan Gui - National Engineering Research Center for Biomaterials, Sichuan University, Chengdu, Sichuan 610065, China
Ke Li - National Engineering Research Center for Biomaterials, Sichuan University, Chengdu, Sichuan 610065, China
Longjian Zhang - National Engineering Research Center for Biomaterials, Sichuan University, Chengdu, Sichuan 610065, China
Li Yang - National Engineering Research Center for Biomaterials, Sichuan University, Chengdu, Sichuan 610065, China; Orcidhttps://orcid.org/0000-0003-0456-4041
Yunbing Wang - National Engineering Research Center for Biomaterials, Sichuan University, Chengdu, Sichuan 610065, China; Orcidhttps://orcid.org/0000-0002-2412-6762
References

发表留言
暂无留言
输入您的留言参与专家互动