Successful closure of a 24mm large atrial septal defect using the MemoSorb biodegradable ASD occluder.
Patient information
Gender: Female
Age: 67 years
Chief Complaint:
The patient has experienced fatigue accompanied by dizziness for six months, with two episodes of syncope.
History of Present Illness:
The patient began experiencing fatigue without obvious triggers six months prior to admission, which worsened with physical activity and was accompanied by a sensation of lightheadedness. Within the past two months, she had two episodes of transient syncope, both preceded by chest pain and followed by transient loss of consciousness.
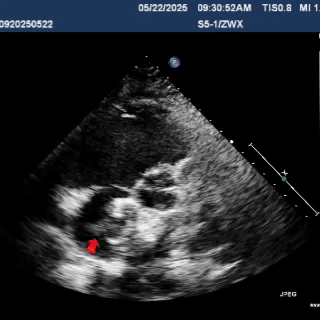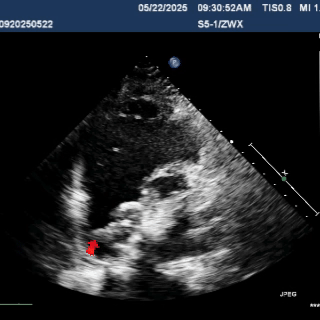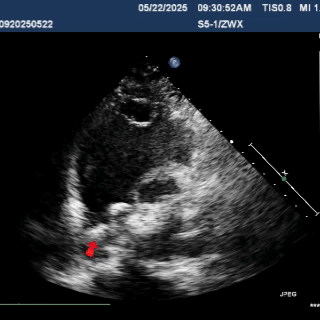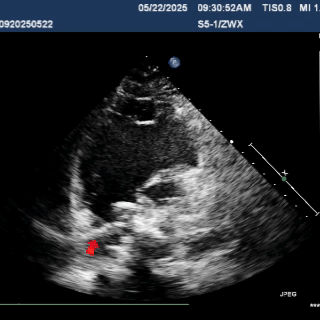
Transthoracic echocardiography indicated a secundum-type atrial septal defect (ASD) measuring 16.9 × 18.7 mm.
The patient generally tolerates physical activity well, with no observed cyanosis of the lips or fingertips. Slight developmental delay was noted. She was admitted for further evaluation and treatment.
Strategy
Device Selection Considerations:
(Type) Secundum atrial septal defect (ASD).
Preoperative measurement after admission showed the defect size to be 16.9 × 18.7 mm.
Initial plan was to use a BDASD-I 26 occluder for closure.
However, intraoperative reassessment revealed that the defect included a soft rim, with a total diameter of 24.1 mm.
Therefore, it was ultimately decided to use a BDASD-I 32 occluder for closure.
Strategy:
Transfemoral percutaneous closure was performed using a BDASD-I 32 occluder and a 16F biodegradable occluder delivery system.
The entire procedure was guided by both ultrasound and digital subtraction angiography (DSA).
Process
Intraoperative reassessment of defect size
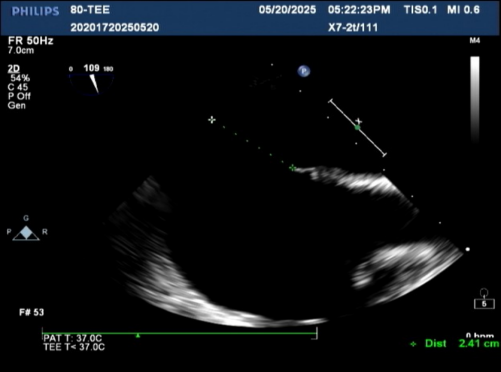
defect size was 24.1 mm
Establishing the delivery pathway
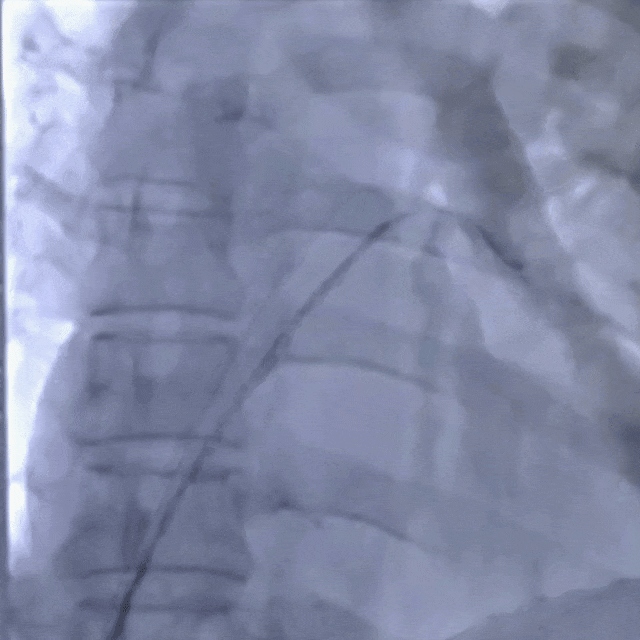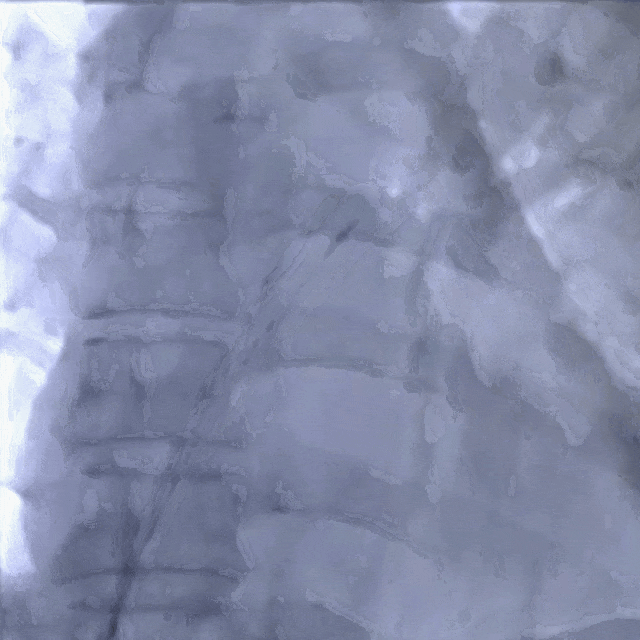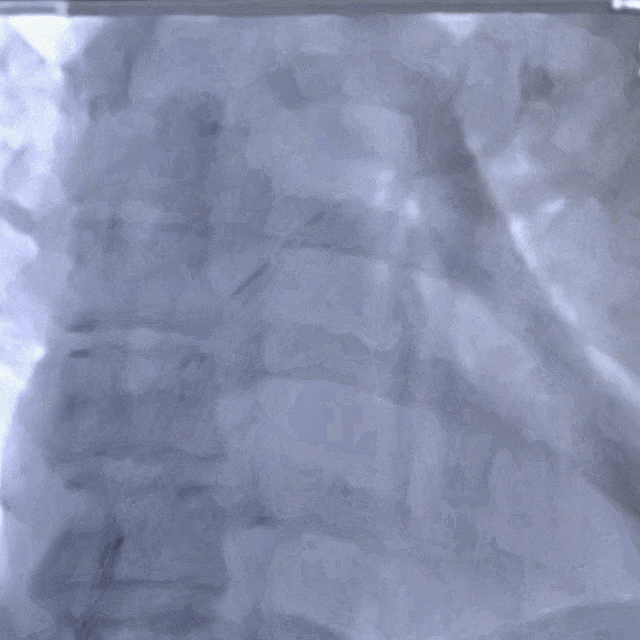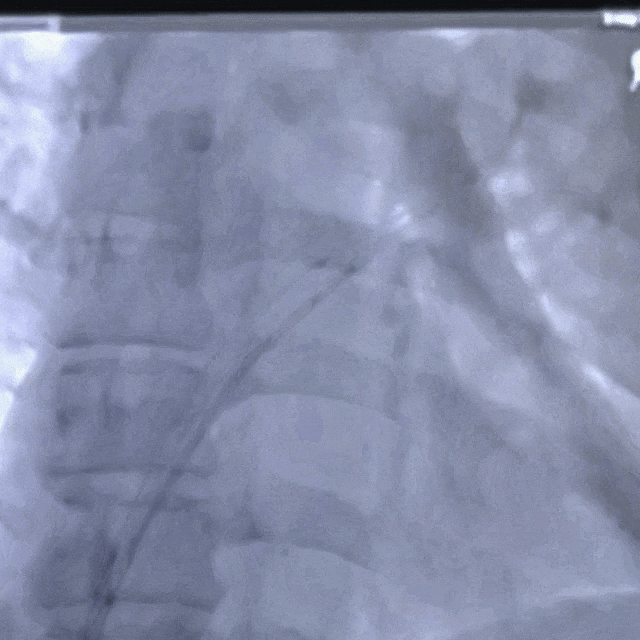
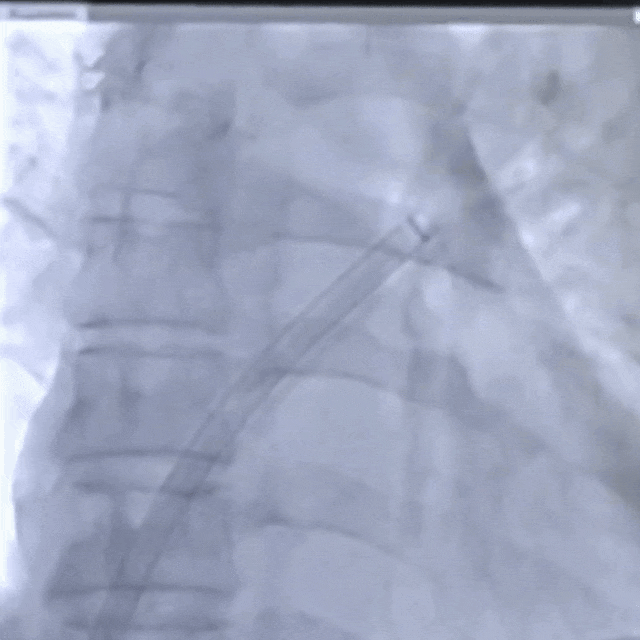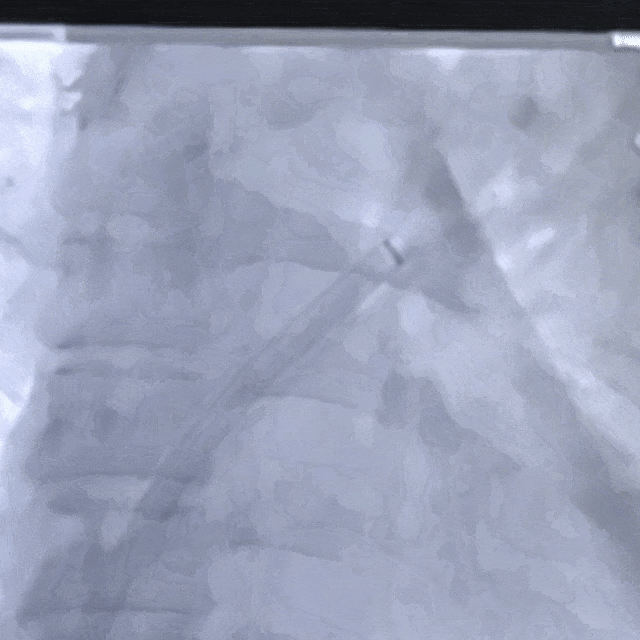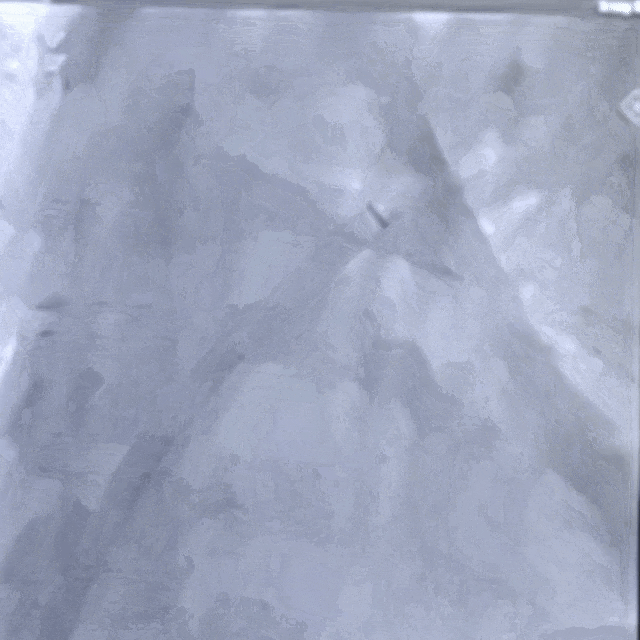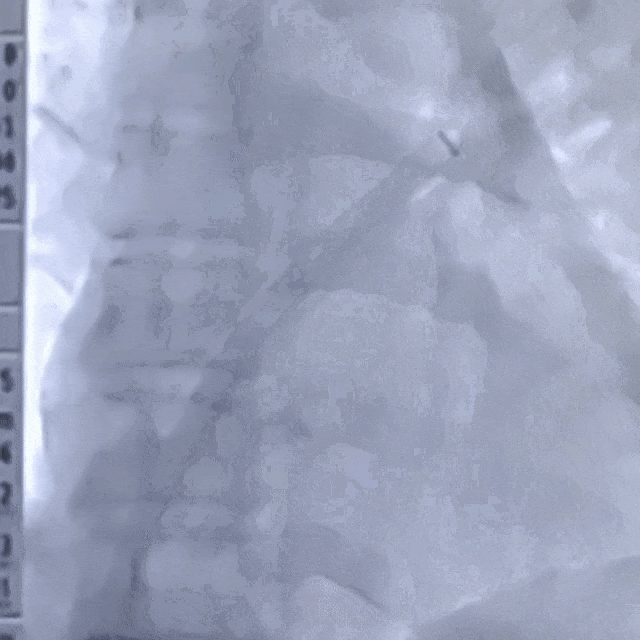
Guidewire placement in the left superior pulmonary vein
The sheath was advanced into the left atrium over the guidewire.
The left disc was deployed and positioned against the atrial septum
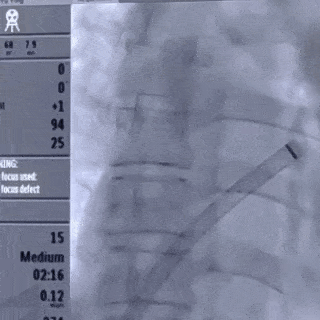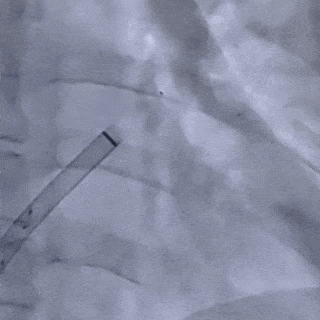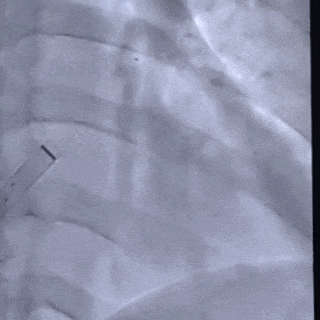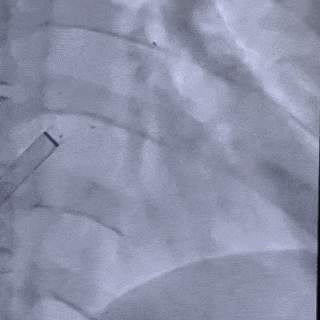
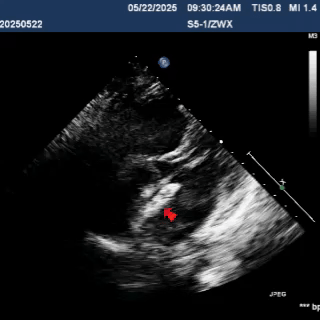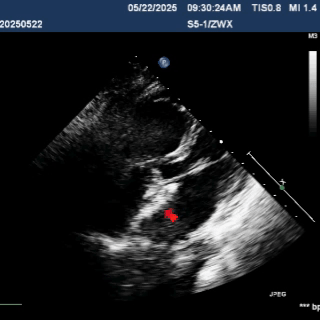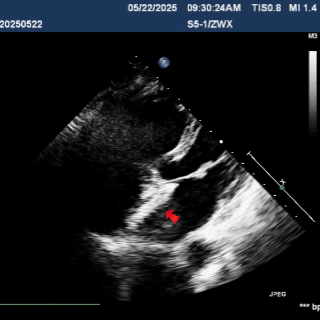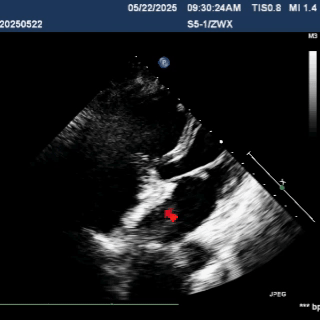
Deliver the left disc
Tension the shaping wire to form the left disc surface. Retract the sheath and steel cable to position the left disc against the atrial septum
Deploy the right disc
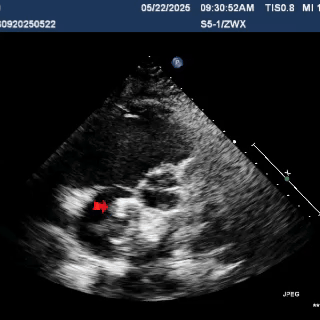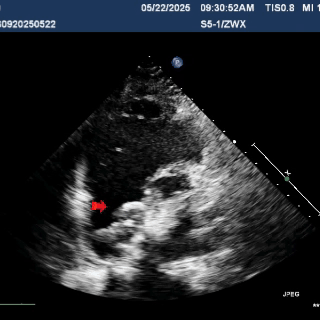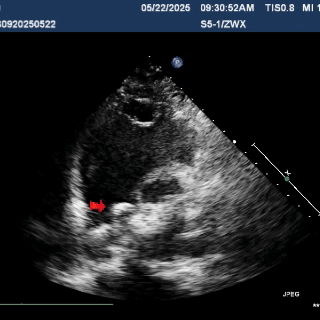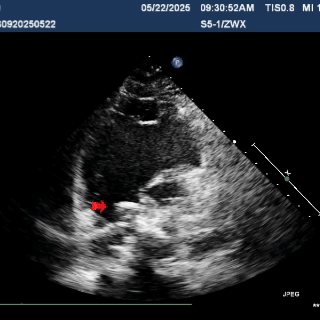
The right disc was gradually released while tensioning the shaping wire to ensure both discs of the occluder clasp the septum firmly
Pull test before locking
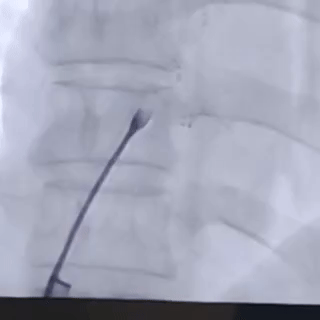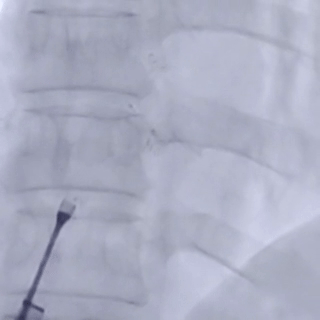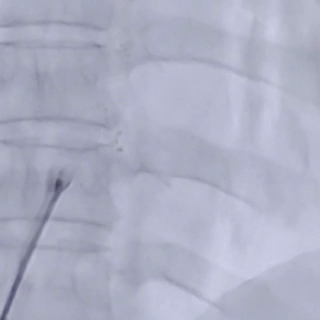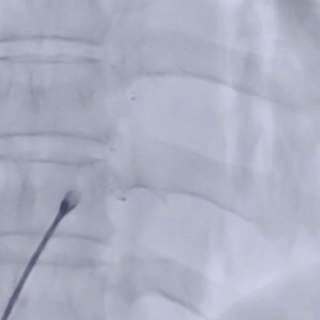
Multiplane ultrasound was used to observe the position and shape of the occluder, confirming that the device straddled both sides of the defect
Under DSA guidance, a tug test was performed on the occluder, during which only the right disc moved in response to the pull
Locking and pull test
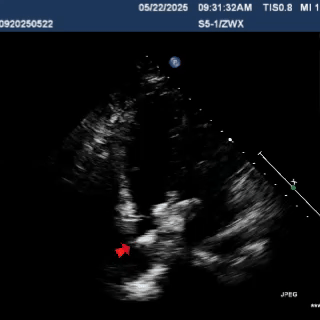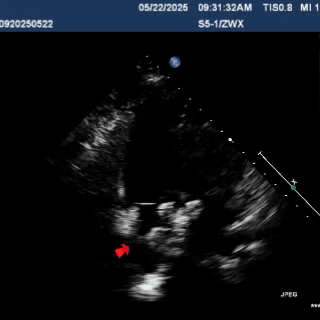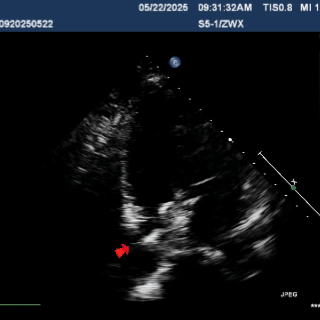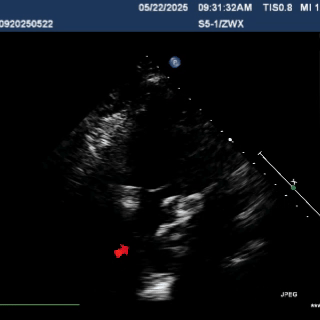
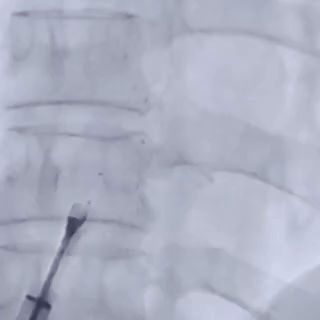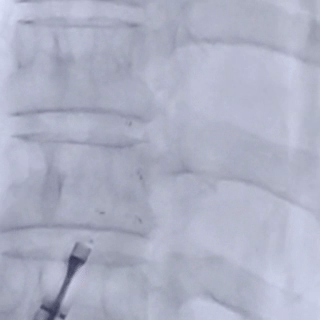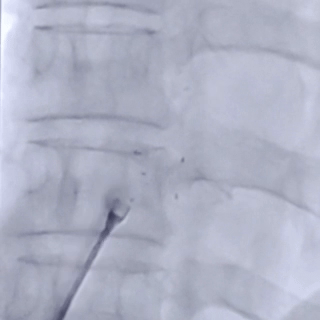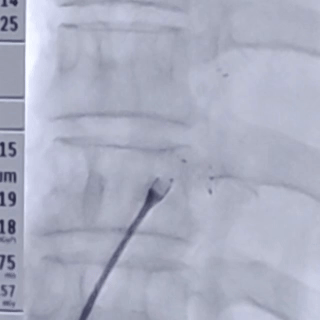
After the sheath contacted the occluder, the front push cable was advanced.
The assistant held the cable and sheath firmly, while tensioning the shaping wire to lock the device.
A tug test was performed, confirming that the occluder straddled both sides of the defect.
The occluder was stable overall and moved as a whole when the cable was pulled
Release the cable, and the occluder is fully deployed
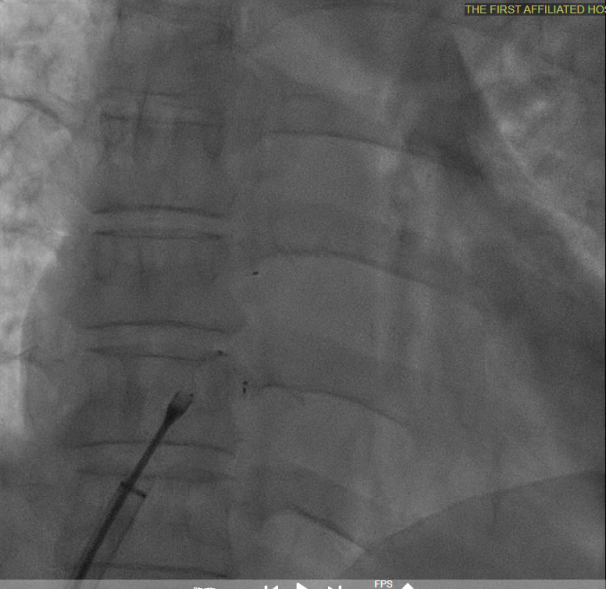
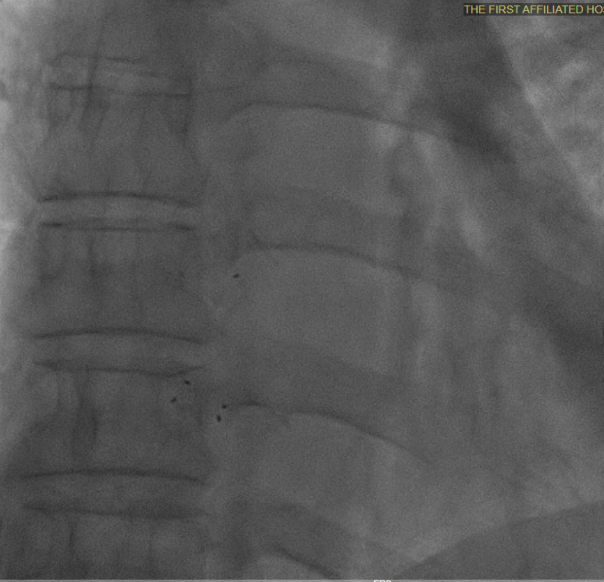
Multiplane observation of the occluder's morphology after deployment.
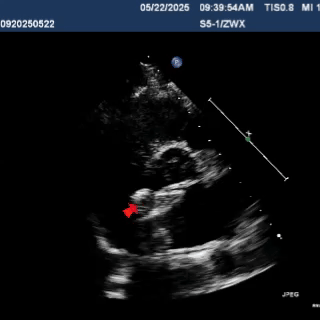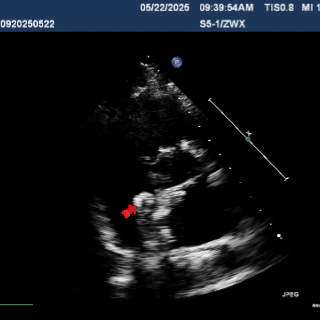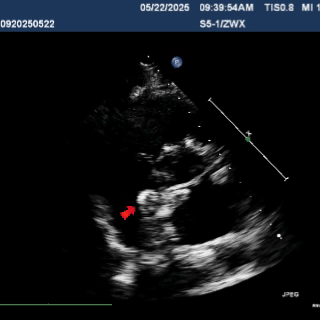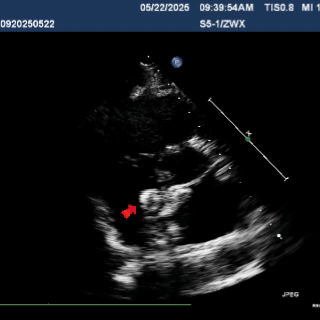
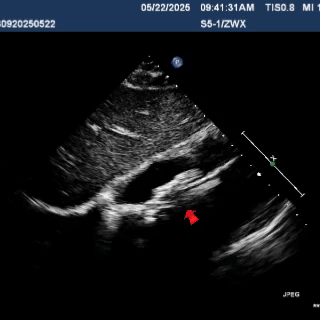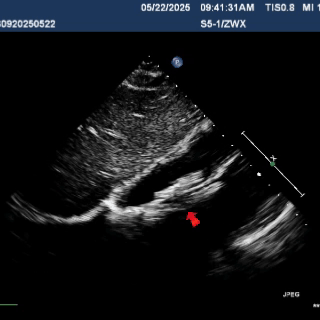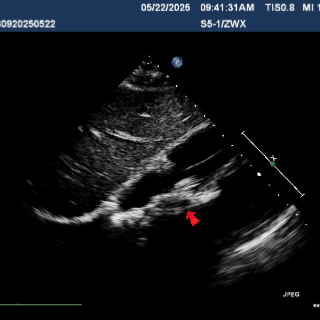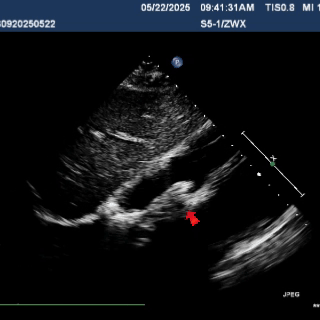
Detach the cable to fully release the occluder.
In the short-axis view of the aorta, the occluder appears Y-shaped, securely embracing the aortic root with stable clamping.
In the subxiphoid four-chamber view, the occluder is observed to be correctly positioned with a good morphology
Summary
Intraoperative reassessment of the atrial septal defect (ASD) size measured 24 mm, with margins measuring 14.2 mm from the inferior vena cava, 15.8 mm from the superior vena cava, and anterior and posterior rims measuring 10.1 mm and 16 mm, respectively. Left-to-right shunting at the atrial level was confirmed.
A BDASD-I 32 occluder and a 16F biodegradable occluder delivery system were selected for the procedure. The ASD closure was performed via a percutaneous transfemoral approach under transthoracic echocardiography (TTE) and digital subtraction angiography (DSA) guidance.
Intraoperative reassessment of ASD size is a critical safety mechanism to ensure procedural success and prevent complications, especially when using biodegradable devices. This necessity arises from the combined effects of anatomical complexity, device characteristics, and dynamic physiological changes. Approximately 30% of ASD patients have deficient rims, and the occluder may shift due to cardiac motion after deployment. Intraoperative measurement of the soft rim size is essential to minimize the risk of intraoperative complications and postoperative residual shunting.
In this case, the defect was relatively large. Using a metal occluder might increase the risk of contact and compression or injury to the aortic root. The biodegradable ASD occluder offers superior wall apposition and conformity, tightly adhering to the defect after locking, which facilitates endothelialization. Additionally, its patented shaping and locking design ensures stable fixation, reducing the risk of device dislodgement, thus demonstrating clear clinical advantages in treating complex and large ASDs.
After deployment of the left and right discs, the short-axis view of the aorta showed the occluder in a Y-shaped configuration embracing the aortic root. Tug testing confirmed stable device positioning without obvious displacement or deformation. Multiplane imaging demonstrated good occluder morphology and no residual shunting. The closure procedure was successful.
Thanks to Dr Peng Feng and the team from the Department of Cardiology, The First Affiliated Hospital of Fujian Medical University, for their case sharing
- End -

发表留言
暂无留言
输入您的留言参与专家互动