Transcatheter closure of perimembranous ventricular septal defect using a novel fully bioabsorbable occluder: multicenter randomized controlled trial
Abstract
Although the use of bioabsorbable occluder is expected to reduce the risk of metal occluder-related complications, it has not been approved due to incomplete degradation and new complications. Novel fully bioabsorbable occluders were designed to overcome such limitations. The aim of this study was to investigate the efficacy and safety of a fully biodegradable occluder in patients with ventricular septal defects. 125 patients with perimembranous ventricular septal defect (VSD) larger than 3 mm were screened from April 2019 to January 2020 in seven centers. 108 patients were enrolled and randomized into the bioabsorbable occluder group (n = 54 patients) and nitinol occluder group (n = 54). A non-inferiority design was utilized and all patients underwent transcatheter device occlusion. Outcomes were analyzed with a 24-month follow-up. All patients were successfully implanted and completed the trial. No residual shunt >2 mm was observed during follow-up. Transthoracic echocardiography showed a hyperechoic area corresponding to the bioabsorbable occluder which decreased primarily during the first year after implantation and disappeared within 24 months. Postprocedural arrhythmia was the only occluder-related complication with an incidence of 5.56% and 14.81% for the bioabsorbable and nitinol groups, respectively (P = 0.112). The incidence of sustained conduction block was lower in the bioabsorbable occluder group (0/54 vs. 6/54, P = 0.036) at 24-month follow-up. In conclusion, the novel fully bioabsorbable occluder can be successfully and safely implanted under echocardiography guidance and reduce the incidence of sustained postprocedural arrythmia. The efficacy and safety of this fully biodegradable occluder are non-inferior to that of a traditional nitinol one.
1. Introduction
Occluders are pivotal devices in the treatment of structural heart disease, including ventricular septal defect (VSD), atrial septal defect, and left atrial appendage. Although nitinol-based occluders are predominantly used in current clinical practice, they have drawbacks such as nickel release, and tissue erosion and compression, which may underlie severe complications such as allergy, cardiac perforation, and complete atrioventricular block [1], [2], [3], [4], [5]. An occluder made of bioabsorbable materials is expected to circumvent these drawbacks based on the premise that the temporary bioabsorbable scaffold would allow endothelization to create a native tissue barrier. However, the development of bioabsorbable occluders faces three main challenges. Firstly, mismatched rate of occluder degradation and endothelization which might result in defect recanalization. In addition, bioabsorbable materials are radiotranslucency and less resilient compared with nitinol. Therefore, bioabsorbable occluders have required a metal frame to strengthen support, maintain occluder shape, and render the occluder visible under fluoroscopy. Unfortunately, the metal frame results in incomplete degradation of the occluder and even cardiac perforation [6], [7], and the addition of metal markers to render occluders visible under fluoroscopy is at risk of dislodging and embolizing during occluder degradation.
In view of these challenges, we designed a new metal-free occluder fully made of bioabsorbable materials. To foster coordinated occluder degradation and endothelization, we chose a hybrid structure, composed of polydioxanone (PDO) monofilament with relatively rapid degradation as double-umbrella framework and poly-L-lactic acid (PLLA) fabric with relatively slow degradation as barrier-film. Moreover, we added a shape line to facilitate framework shaping into a double-umbrella and recover support strength after the occluder is released from the delivery sheath. What’s more, echocardiography is used for procedural guidance in lieu of fluoroscopy, and provides good visibility of the bioabsorbable occluder thereby rendering a metal frame or markers unnecessary.
The complications associated with the use of a metal occluder for perimembranous VSD closure have been the subject of controversy and precluded its approval for clinical use in North America. We therefore conducted research and applied the abovementioned innovative design features to a bioabsorbable occluder for VSD closure. The short-term outcomes of this fully bioabsorbable occluder for perimembranous VSD were favorable based on preliminary analysis in five patients [8]. In this multicenter, randomized, controlled trial, we aimed to further assess the efficacy and two-year safety of the novel fully bioabsorbable occluder.
2. Materials and methods
2.1. Study design and patients
This trial was a prospective, multicenter, randomized study of the novel occluder in the treatment of perimembranous VSD (ClinicalTrials.gov, number NCT03941691). Patients were recruited at seven hospitals in China. All eligible patients had to meet the following inclusion criteria: perimembranous VSD with a diameter ranging from 3 to 14 mm; age ranging from 1 to 60 years; weight >10 kg; and distance between the edge of defect and the aortic valve >3 mm. Patients with any of the following conditions were excluded: other cardiac malformation dependent on VSD or requiring surgery; severe pulmonary hypertension with bidirectional shunt or right-to-left shunt; aortic valve prolapse; or moderate or severe aortic regurgitation.
Patients were initially screened for study eligibility by transthoracic echocardiogram (TTE). If the patient met all inclusion criteria, the patient or the legal guardian was informed about the study and signed the informed consent to participate in this study. Following general anesthesia in the operating room, transesophageal echocardiography (TEE) was performed to assess VSD anatomy for eligibility. The patient was then identified as a suitable candidate for VSD device closure and registered in the study. The registered patients were randomized 1:1 to VSD closure with either bioabsorbable occluder or nitinol occluder. The excluded patients after the TEE assessment underwent surgical repair. All patients who received device closure underwent follow-up at discharge, 30 days, 6 months, 12 months, and yearly thereafter.
The trial was approved by the ethics committees of the seven centers (approval No. of the leading center, 2018-1077). All procedures performed in studies involving human participants were in accordance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Written informed consent was obtained from all patients/guardians.
2.2. Sample size and randomization
The sample-size calculations were based on the expectation that device closure for VSD using this novel bioabsorbable occluder would be noninferior to using traditional nitinol occluder in successful closure of the defect, defined as successful implantation of the occluder and no or <2 mm residual shunt at 6 months postoperatively. According to the experience of clinicians, it was assumed that the success rate of defect closure using the nitinol occluder is 98% and the non-inferior margin is clinically acceptable as 8%. Assuming a one-sided type I error rate of 2.5% and 80% power and considering a dropout rate of 10%, the sample size was calculated to be 54 for each group.
2.3. Devices and procedure
The fully bioabsorbable VSD occluder, produced by Shanghai Shape Memory Alloy Ltd (Shanghai, China), consists of a symmetric “double-umbrella” framework woven by PDO monofilament (molecular mass, 200 kDa; diameter, 0.15 mm for sizes 4–7 or 0.20 mm for sizes 8–16) with PLLA(molecular mass, 100 kDa) non-braided fabric filled in both disks, which finally hydrolyze into carbon dioxide and water via the Krebs cycle [9], [10]. The occluder is self-expandable, reclaimable, and can be delivered through a sheath. Because the elasticity of PDO is worse than that of nitinol, the occluder shaping is aided by a shape line, which is tied on the center of the left disc and has a knot on the other end. A connected line is linked with the shape line through the knot. The occluder is available in sizes of 4–16 mm in waist diameter, and correspondingly delivered through 8–10 French (Fr) sheath. The delivery system is made up of a stainless-steel spring tube with a clamp on the tip (Fig. 1). The metal occluder used in this study is symmetric with two disks and a waist woven by nitinol wire, and it is also produced by Shanghai Shape Memory Alloy Ltd. The details of this occluder have been reported previously [11].
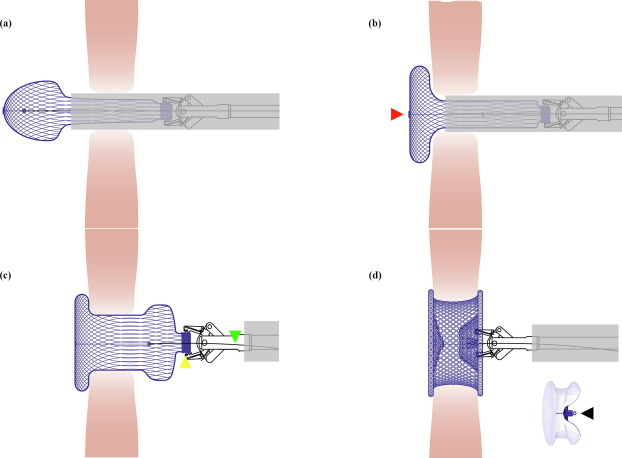
Fig. 1. Illustration for the occluder shaping process. (a) The left side of the bioabsorbable occlude was spherical after being released from the delivery sheath. (b) The left side of the bioabsorbable occluder was shaped from spherical shape to disc shape by pulling back the shape line, which was tied on the center of the left disc (indicated by the red triangle). (c) The right side was spherical after being released from the delivery sheath. The yellow triangle indicated the connecting rivet of the occluder. The green triangle indicated the connecting line. (d) The entire occluder was shaped into the “double-umbrella” framework by pulling back the shape line and simultaneously pushing the delivery cable. There was a knot (indicated by the black triangle) on the shape line, which would be pulled beyond the connecting rivet of the occluder, in order to keep the double-umbrella shape stable after releasement.
The implantation procedure of the bioabsorbable occluder was similar to that of the nitinol occluder [11]. In the present study, the occluder was implanted through a perventricular approach under TEE guidance. The procedure was performed in an operating room, and the patient was under general endotracheal anesthesia. The right ventricular free wall was exposed via a minimal inferior sternotomy, a purse-string suture was placed on the coronary-free area of the right ventricular free wall, and a trocar was inserted into the right ventricular cavity through the purse-string. Under TEE guidance, a 0.035-inch guide wire was introduced into the left ventricle via the defect through the trocar. After the trocar was withdrawn, a delivery sheath was advanced over the guide wire via the defect into the left ventricle, and then the inner sheath and guide wire were removed under TEE guidance. The occluder selected was 1–2 mm larger than the largest defect diameter measured by TEE, and then it was delivered through the prepared loading sheath and delivery sheath. There was one more step of occluder shaping in the release process of the bioabsorbable occluder. The left disc of the bioabsorbable occluder needed to be shaped from spherical shape to disc by pulling back the shape line by the connecting line after the occluder was released from the sheath. After deployment of the right disc, the entire occluder was shaped into the “double-umbrella” framework by pulling back the shape line and simultaneously pushing the delivery cable. When the knot of the shape line was pulled out of the connecting rivet of the occluder, the occluder was locked into a “double-umbrella” shape (Fig. 1, Video S1 online). The connecting line was then removed from the occluder. TEE was used to reassess occluder shape and position, the presence of a residual shunt, and valvular regurgitation before and after occluder deployment. Thereafter, the shape line and delivery sheath were removed, and the purse-string suture was tied. All patients were transferred to the intensive care unit, where the endotracheal tube was removed. All patients were prescribed daily aspirin (5 mg/kg) for 6 months post procedure.
2.4. Outcomes and measures
The efficacy endpoint was the rate of successful implantation of the occluder and no or less than 2 mm residual shunt assessed by TTE at 6 months postoperatively. The safety endpoint was the rate of occluder-related complications within 24 months, defined as the composite of death, occluder displacement, new-onset more than mild or aggravated valve regurgitation, cardiovascular surgery for adverse events, symptoms of embolization, or arrhythmias.
2.5. Echocardiographic analysis of occluder biodegradation
Before discharge and at 1, 3, 6, 12, and 24 months after the operation, the areas of the left and right discs in both five-chamber and short-axis views of TTE were measured by dynamic analysis of the region of interest using QLAB quantitative analysis software (Philips Medical, Andover, USA).
2.6. Statistical analysis
Baseline evaluation was assessed in the full analysis set (FAS), which included a set of subjects characterized by the intention-to-treat principle and consisted of all patients who were randomly assigned to receive operation with the study device. The primary efficacy analysis was conducted using the per-protocol set (PPS), which comprised a subset of subjects whose compliance with the protocol was sufficient to ensure that their clinical data would likely exhibit the true effects of treatment according to the protocol. Safety assessment was conducted using the safety set (SS) comprised of a set of subjects receiving bioabsorbable occluder implantation and at least one safety estimation. Data are presented as mean ± standard deviation (SD), median with range, or frequencies and/or percentages. SPSS 26.0 (SPSS, Inc., Chicago, USA) was used for the statistical analysis. The Student’s t test, Welch’s t test, Chi-square test, Fisher’s exact test, and non-parametric test were used for comparison between the two groups, where appropriate. The analyses were based on the intention-to-treat principle. All tests of significance were 2-sided, and a P <0.05 was considered statistically significant.
3. Results
3.1. Participants
From April 2019 to January 2020, 125 patients were screened by TTE, and 8 patients were excluded because of anatomy ineligibility (distance between the edge of defect and the aortic valve <3 mm in 6; multiple perimembranous VSD in 2). One hundred seventeen patients signed the informed consent, and one patient withdrew consent before the procedure. Eight cases were not registered due to disqualification of TEE examination after anesthesia, 2 cases of short distance from the aortic valve, 4 cases of multiple perimembranous VSD, and 2 cases of tricuspid valve and chordae tendineae partial coverage defects. Finally, 108 registered patients were randomly assigned to the bioabsorbable occluder group (n = 54 patients) and the nitinol occluder group (n = 54 patients) (Fig. 2). The median age was 4 (range 1 to 38) years in the bioabsorbable occluder group and 4 (range 1 to 49) years in the nitinol group, respectively (P = 0.680). The median defect size was 4.00 (range 3.00 to 8.20) mm and 4.00 (range 3.00 to 7.50) mm, respectively (P = 0.807). The distribution of baseline clinical characteristics was similar between the two study groups (Table 1).
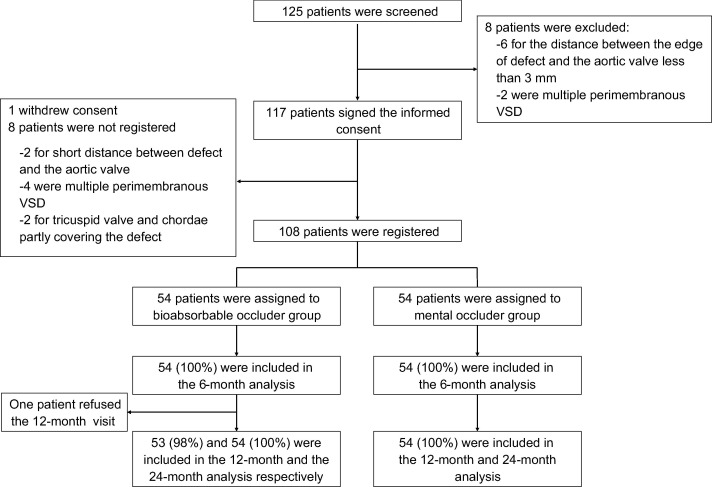
Fig. 2. Study flowchart.
Table 1. Baseline characteristics and perioperative results.
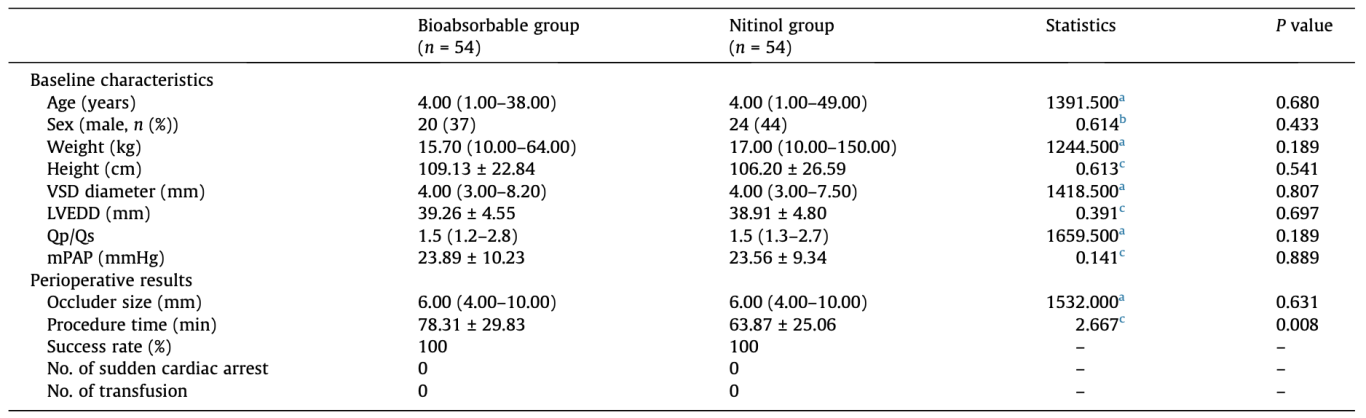
Values are presented as mean ± SD, median (range), n (%), or otherwise noted. LVEDD: left ventricular end-diastolic dimension; Qp/Qs: pulmonary-to-systemic flow ratio; mPAP: mean pulmonary artery pressure.
-
a
U value, independent-samples Mann-Whitney U test.
-
b
Pearson χ2 value, Chi-square test.
-
c
t value, independent-samples t test.
3.2. Perioperative results
All patients in both groups successfully completed device closure for perimembranous VSD. The median size of the occluder was 6.00 (range 4.00 to 10.00) mm in both bioabsorbable and metal groups. The mean procedure time defined as the duration from thoracotomy to sternal closure was 78.31 ± 29.83 min in the bioabsorbable group, and 63.87 ± 25.06 min in the nitinol group (P = 0.008), respectively (Table 1). Residual shunt <2 mm was found in 3 (5.56%) patients of the bioabsorbable group and 1 (1.85%) patient of the nitinol group (P = 0.745), respectively(Table 2). There was no aggravation of aortic or tricuspid regurgitation after occluder implantation in either group. Left ventricular size decreased after surgery in both groups. No major adverse events such as cardiac arrest occurred in either group, and no patient required blood transfusion due to blood loss.
Table 2. Echocardiography results.
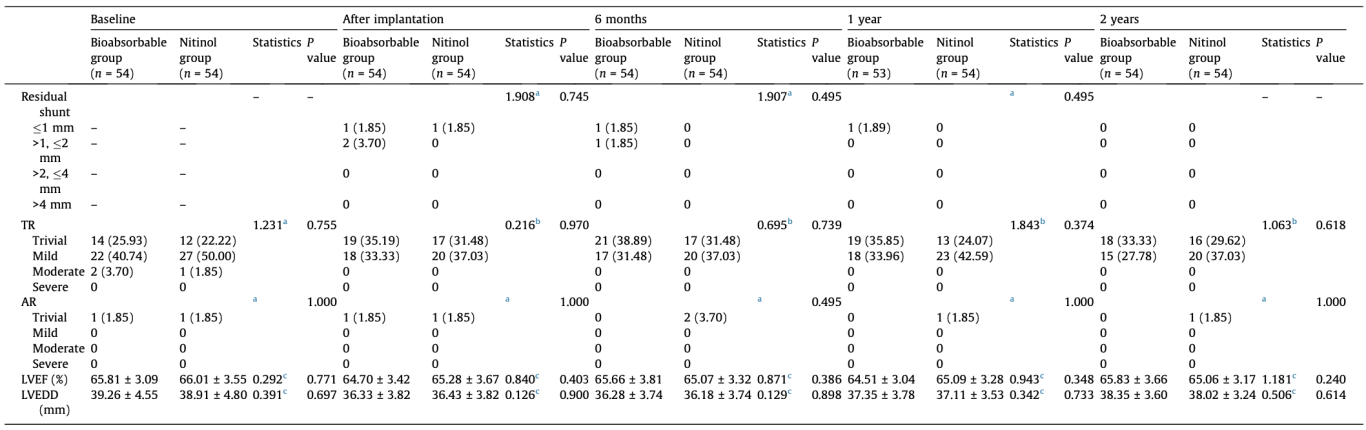
Values are mean ± SD or n (%). Group differences have been evaluated with Welch’s t test or non-parametric test for continuous variables and Pearson’s Chi-square test for categorical variables. AR: aortic regurgitation; TR: tricuspid regurgitation; LVEF: left ventricular ejection fractions.
-
a
Fisher’s exact test.
-
b
Pearson χ2 value, Chi-square test.
-
c
t value, independent-samples t test.
3.3. Follow-up results
3.3.1. Efficacy endpoints
Only one patient in the bioabsorbable occluder group did not attend follow-up visit at 12 months postoperatively. All the patients in both groups completed 6- and 24-month visits. At 6 months, no <2 mm residual shunt was detected by TTE in either group. Therefore, the efficacy endpoint was achieved in 100% of patients in both groups. The difference in the success rate between these two groups was 0 (95% CI: −0.066 to 0.066). The absolute value of the lower limit of the 95% CI was 0.066%, which was less than the specified non-inferiority margin of 8%; therefore, the non-inferiority conclusion was valid. During the 24-month follow-up period, less than 2 mm residual shunt was detected immediately after the procedure disappeared and no new trans-ventricle shunt occurred in both groups.
3.3.2. Safety endpoints
During the 24-month follow-up period, postoperative arrhythmias were the only device-related complications in both groups. The incidence of device-related complications before discharge was 5.56% (3/54) and 14.81% (8/54) in the bioabsorbable and nitinol group, respectively (P = 0.112). In the bioabsorbable group, the three patients developed complete right bundle branch block (RBBB). In the nitinol group, the eight patients had different postoperative arrhythmias. Among them, RBBB occurred in 3 (5.56%) patients, complete left bundle branch block (LBBB) in 2 (3.70%) patients and left anterior fascicular block in 1 (1.85%) patient, frequent premature ventricular contractions in 2 (3.70%) patients. It is worth noting that the conduction block that developed in the three patients with bioabsorbable occluder was reversible (Fig. S1 online) but it was sustained in patients with nitinol occluder at 24 months follow-up. Therefore, when comparing 24-month ECG results between the two groups, the incidence of sustained conduction block was lower in the bioabsorbable occluder group (0/54 vs. 6/54, P = 0.036). No complete atrioventricular block developed in either group during the 24-month follow-up period.
There was no occluder-induced valve dysfunction in either group. In terms of the tricuspid valve, 2 patients in the bioabsorbable group and 1 in the nitinol group had moderate regurgitation before the procedure, which reduced to mild or less after occluder implantation. The trend of tricuspid regurgitation improvement was observed in both groups after VSD closure, but without statistically significant difference. In terms of aortic valve, no mild or more regurgitation was detected in either group during follow-up. Few patients had trivial regurgitation with no significant difference between the two groups.
No other complications including occluder displacement, embolization, abnormal liver and renal function (Table S1 online), surgery, and death occurred in either group during the follow-up.
3.4. Degradation of the bioabsorbable occluder
The degradation process of bioabsorbable occluders was assessed using echocardiography. The bioabsorbable occluder was shown on the echocardiogram as a hyperechoic region that protruded from the surrounding endocardium at 3 months but was at the same level as the surrounding endocardium at the 12-month follow-up. However, no hyperechoic region was detected on the ventricular septum by echocardiography at the 24-month follow-up (Fig. 3). The degradation process was quantified as a reduction in the area of the hyperechoic region, which was demonstrated in both the left and right discs of the biodegradable occluder. In TTE five-chamber view, the area of the left disc decreased from 62.74 ± 5.54 mm2 (measured before discharge) to 6.67 ± 6.64 mm2 (measured at 12 months), while the right disc decreased from 64.42 ± 6.21 mm2 to 18.75 ± 4.08 mm2. In TTE short axis view, the area of the left disc decreased from 64.67 ± 6.16 mm2 (measured before discharge) to 7.01 ± 2.62 (measured at 12 months), and the right disc decreased from 67.56 ± 5.19 mm2 to 15.64 ± 3.65 mm2. The ratio of the hyperechoic region area measured at 1-, 3-, 6-, and 12-month follow-up to that measured before discharge was 93.42%, 81.94%, 63.07%, and 10.69% (mean value of the five-chamber view and short-axis view) for the left disc, and 94.76%, 86.38%, 74.54 %, and 26.17% for the right disc, respectively (Fig. 4).
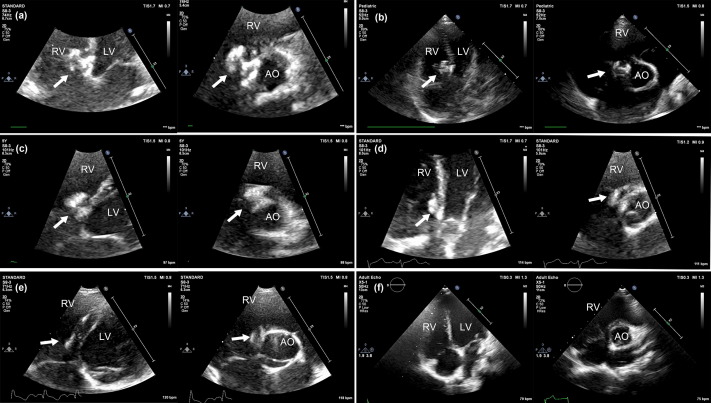
Fig. 3. Morphology changes of the fully bioabsorbable occluder as shown by TTE throughout the 24-month follow-up. (a–f): TTE five-chamber view and short-axis view were examined before discharge, and at 1-, 3-, 6-, 12-, and 24-month follow-up, respectively. Arrows indicate the occluder. RV: right ventricle; LV: left ventricle; AO: ascending aorta.

Fig. 4. Quantitative analysis in the area of the fully bioabsorbable occluder as shown by TTE throughout the 24-month follow-up. (a) The area measured in a five-chamber view. (b) Ratio of the disc area measured at 1-, 3-, 6-, and 12-month follow-up to that measured before discharge in five-chamber view. (c) The area measured in a short-axis view. (d) Ratio of the disc area measured at 1-, 3-, 6-, and 12-month follow-up to that measured before discharge in short-axis view. *P <0.05. Error bar shows standard error of the mean.
4. Discussion and conclusion
Interventional technology is one of the greatest advances in the cardiovascular field, and occluders and stents are the most widely used implants. However, the use of metal devices is associated with long-term complications such as cardiac erosion, restenosis, and compression. Devices made of bioabsorbable materials are expected to avoid these events. Unfortunately, previous bioabsorbable occluders had a mismatched rate of degradation and endothelization, incomplete degradation, and lesser resilience, which led to new complications. Therefore, bioabsorbable occluders have not yet been recognized and widely used. Therefore, we designed a novel fully bioabsorbable occluder with improvements to address the latter limitations. In this study, the efficacy and safety of this novel occluder were compared with those of the traditional nitinol occluder, and the therapeutic effect of the biodegradable device was noninferior to that of the traditional device.
The first challenge for an occluder made of only bioabsorbable materials is how to safely implant it into the heart. Several studies have documented the safety and efficacy of echocardiography-guided closure of septal defect by transfemoral or transthoracic approaches [11], [12], [13], [14]. The framework woven by PDO filament has good visibility by echocardiography, enabling the occluder to be implanted under echocardiographic guidance. Therefore, the change in guiding image modality from fluoroscopy to echocardiography allows the implantation of a fully bioabsorbable occluder without any metallic component.
Compared with well-established surgical repair of perimembranous ventricular septal defects, transcatheter occlusion was proofed to provide a less invasive treatment with equal efficacy [15]. Although this fully bioabsorbable occluder is designed to be implanted via transfemoral or transthoracic route, the use of transthoracic perventricular approach was used in this trial in compliance with ethics committee requirements to provide maximum patient safety. Because the present trial is the first in the world to evaluate a fully bioabsorbable occluder which is completely invisible under X-ray, transthoracic device closure by inferior mini sternotomy can be immediately converted to routing open-heart surgery, to prevent severe complications during the implantation process.
Since the resilience of the PDO framework is not as good as that of the nitinol one, another challenge is making the bioabsorbable occluder return to a double-umbrella shape after it is released from the delivery sheath. An innovative solution is to add a wire to the left disc, which provides a counterforce to the delivery cable, allowing the occluder to be easily and effectively shaped into a double umbrella. This occluder shaping step required additional time compared with nitinol occluder implantation, which explains the longer procedure time in the bioabsorbable occluder group.
A key desired feature of a bioabsorbable occluder is that it provides adequate support and blocks the defect until endothelization is completed, i.e., for the native tissue to completely cover the defect. Complete endothelization of the disc surface has been reported to take 3–6 months [11], [20]. The PDO framework sustained the dormant stage for at least 90 days and then entered into the active degradation stage [16]. The PDO-based framework provides enough strength and then rapidly degrades after endothelization. Degradation of the PLLA fabric is significantly slower than that of the PDO framework. The PLLA fabric was partially degraded at 6 months thereby preventing postoperative VSD recanalization [17], [18]. Therefore, this novel “double-umbrella” occluder composed of PDO framework and PLLA fabric can degrade completely and close the defect without recanalization.
The degradation process of the bioabsorbable occluder can be assessed semiquantitatively by echocardiography. The double-umbrella shape of the occluder can be detected in five-chamber and short-axis views as a hyperechoic area protruding from the surrounding endocardium within 6 months, which suggested that the occluder still retained sufficient strength to close the defect. At the 12-month echocardiographic follow-up, the hyperechoic area was flush with the surrounding endocardium, indicating that the occluder degraded primarily during this timeframe. No new trans-ventricle shunt occurred during follow-up in the patients receiving the bioabsorbable occluder, suggesting an appropriate balance between endothelization and degradation. Furthermore, the decline rate of the hyperechoic area in the left disc region was significantly higher than that in the right disc region, suggesting that the left disc degraded faster than the right disc possibly due to faster blood flow and higher pressure in the left ventricle than in the right ventricle. This phenomenon was not recorded in other studies of bioabsorbable atrial septal occluder [19], [20], [21], because of the similar pressure in the two atria. Divergent degradation of various parts of the occluder should not be ignored when developing new bioabsorbable occluders for other diseases. For example, bioabsorbable occluder for patent ductus arteriosus may migrate, resulting from faster degradation of the aortic side than the pulmonary artery side. Therefore, different sides of the occluder should be made with materials with different molecular weights, in order to keep balanced degradation of the two sides. In the electrocardiographic assessment at 24 months in the present study, the bioabsorbable occluder group had a significantly lower incidence of sustained conduction block. This could be ascribed to the ability of the bioabsorbable occluder to completely degrade. Cardiac conduction block is the main concern in device closure of perimembranous VSD [5]. Although the exact mechanism of conduction block following device closure remains unclear, subsequent compression stress on the surrounding septal tissue and subsequent stimulus reaction induced by the device are considered to play important roles in the development of conduction block. The reversible complete RBBB recorded in the bioabsorbable occluder group may be explained by a reduction or disappearance of pressure and stimulus reaction due to occluder degradation. Although the fatal late-onset complication of CABV did not occur in either group during follow-up, the bioabsorbable occluder degrades completely after endothelization without any residue, which promises to minimize or eliminate the risk of late-onset complications.
Inevitably, the current study had some potential limitations, including a rather small sample size for each group. Thus a larger study with longer follow-up in the future is warranted. In addition, histological examination which is routinely conducted in animal studies was not used in this human study to evaluate the extent of occluder degradation and endothelization, however, VSD recanalization did not occur which indicates that endothelization was completed before complete device degradation. The bioabsorbable occluder group had a salutary effect on the incidence of sustained bundle branch block and ventricular premature beat, despite that at the 2-year follow-up, the bioabsorbable occluder was not better at preventing cardiac erosion and complete atrioventricular block, which did not occur in either group.
In summary, this novel fully bioabsorbable occluder with a hybrid structure combining PDO and PLLA can be successfully and safely implanted with the help of echocardiography and the shape line, and effectively closes perimembranous VSDs. Our result indicated that the efficacy of this fully biodegradable occluder is non-inferior to that of a traditional nitinol one. Besides, in terms of safety endpoint, the bioabsorbable occluder degrades completely thereby reducing the risk of sustained postprocedural arrhythmia. Improvements including hybrid structure, shape line, and echocardiographic guidance, as well as a caution about divergent degradation pave the way for the development of new generation bioabsorbable occluders for other structural heart diseases.
Conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgments
This work was supported by the National Key Research and Development Program (2022YFC2503400), the Fundamental Research Funds for the Central Universities (2019PT350005), the National Natural Science Foundation of China (81970444), the Beijing Municipal Science and Technology Project (Z201100005420030), the National High Level Talents Special Support Plan (2020-RSW02), the Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (2021-I2M-1-065), and the Sanming Project of Medicine in Shenzhen (SZSM202011013).
Author contributions
Shouzheng Wang, Zefu Li, and Yunbing Wang conceived the trial and wrote the manuscript. Tianli Zhao, Xuming Mo, Taibing Fan, Jianhua Li, Tao You, Gianfranco Butera, and Ziyad M. Hijazi performed the clinical trial and analyzed the data. Rundi Deng, Wenbin Ouyang, Weiwei Wang, Chuangnian Zhang, Zhiling Luo, and Xiangbin Pan conceived the methodology. Kunjing Pang, Da Zhu, Shiliang Jiang, Gejun Zhang, Xiaopeng Hu, and Yongquan Xie collected and visualized the result data. Fengwen Zhang, Fang Fang, Jingping Sun, Ping Li, and Juan Chen supervised the investigation. Zhiling Luo and Xiangbin Pan reviewed and edited the manuscript. All authors approved the final version of the manuscript.
[1]B. Bahrani, N. Moghaddam, J. DeKoven
Cross-sectional survey of nickel allergy management in the context of intracardiac device implantation
Dermatitis, 30 (2019), pp. 213-221
[2]V. Sharma, R.A. DeShazo, C.R. Skidmore, et al.
Surgical explantation of atrial septal closure devices for refractory nickel allergy symptoms
J Thorac Cardiovasc Surg, 160 (2020), pp. 502-509
[3]A. Divekar, T. Gaamangwe, N. Shaikh, et al.
Cardiac perforation after device closure of atrial septal defects with the amplatzer septal occluder
J Am Coll Cardiol, 45 (2005), pp. 1213-1218
[4]P. Tsinivizov, A. Giannakopoulos, D. Varvarousis, et al.
Cardiac tamponade due to very late perforation of left atrium by atrial septal defect occluder
JACC Cardiovasc Interv, 14 (2021), pp. e49-e51
[5]M. Carminati, G. Butera, M. Chessa, et al.
Transcatheter closure of congenital ventricular septal defects: results of the European Registry
Eur Heart J, 28 (2007), pp. 2361-2368
[6]C.M. Happel, K.T. Laser, M. Sigler, et al.
Single center experience: Implantation failures, early, and late complications after implantation of a partially biodegradable ASD/PFO-device (BioStar®)
Catheter Cardiovasc Interv, 85 (2015), pp. 990-997
[7]M. Cikirikcioglu, S. Cherian, R. Lerch, et al.
Late tamponade secondary to aortic root perforation by biostar septal closure device
Ann Thorac Surg, 91 (2011), pp. 604-606
[8]L. Chen, S. Hu, Z. Luo, et al.
First-in-human experience with a novel fully bioabsorbable occluder for ventricular septal defect
JACC Cardiovasc Interv, 13 (2020), pp. 1139-1141
[9]J.C. Middleton, A.J. Tipton
Synthetic biodegradable polymers as orthopedic devices
Biomaterials, 21 (2000), pp. 2335-2346
[10]J.P. Oberhauser, S. Hossainy, R.J. Rapoza
Design principles and performance of bioresorbable polymeric vascular scaffolds
EuroIntervention, 5 (Suppl F) (2009)
F15-22
[11]W.B. Ou-Yang, S.J. Li, S.Z. Wang, et al.
Echocardiographic guided closure of perimembranous ventricular septal defects
Ann Thorac Surg, 100 (2015), pp. 1398-1402
[12]W.-B. Ou-Yang, S.-Z. Wang, S.-S. Hu, et al.
Perventricular device closure of perimembranous ventricular septal defect: effectiveness of symmetric and asymmetric occluders
Eur J Cardiothorac Surg, 51 (2017), pp. 478-482
View at publisher
[13]S. Wang, W. Ouyang, Y. Liu, et al.
Transcatheter perimembranous ventricular septal defect closure under transthoracic echocardiographic guidance without fluoroscopy
J Thorac Dis, 10 (2018), pp. 5222-5231
[14]H. Bu, Y. Yang, Q. Wu, et al.
Echocardiography-guided percutaneous closure of perimembranous ventricular septal defects without arterial access and fluoroscopy
BMC Pediatr, 19 (2019), p. 302
[15]J. Yang, L. Yang, S. Yu, et al.
Transcatheter versus surgical closure of perimembranous ventricular septal defects in children: a randomized controlled trial
J Am Coll Cardiol, 63 (2014), pp. 1159-1168
[16]M.A. Sabino, J. Albuerne, A.J. Muller, et al.
Influence of in vitro hydrolytic degradation on the morphology and crystallization behavior of poly(p-dioxanone)
Biomacromolecules, 5 (2004), pp. 358-370
[17]R. Fitzgerald, L.M. Bass, D.J. Goldberg, et al.
Physiochemical characteristics of poly-L-lactic acid (PLLA)
Aesthet Surg J, 38 (2018), pp. S13-S17
[18]Y.F. Zhu, X.M. Huang, J. Cao, et al.
Animal experimental study of the fully biodegradable atrial septal defect (ASD) occluder
J Biomed Biotechnol, 2012 (2012), Article 735989
[19]O. Baspinar, M. Kervancioglu, M. Kilinc, et al.
Bioabsorbable atrial septal occluder for percutaneous closure of atrial septal defect in children
Tex Heart Inst J, 39 (2012), pp. 184-189
[20]K. Sievert, S. Bertog, B. Soderberg, et al.
Transcatheter closure of atrial septal defect and patent foramen ovale with carag bioresorbable septal occluder: first-in-man experience with 24-month follow-up
EuroIntervention, 17 (2021), pp. 1536-1537
[21]D. Pavcnik, K. Tekulve, B.T. Uchida, et al.
Double biodisk: a new bioprosthetic device for transcatheter closure of atrial septal defects – a feasibility study in adult sheep
Radiol Oncol, 46 (2012), pp. 89-96

Shouzheng Wang is a cardiac surgeon at the Department of Structural Heart Disease, Fuwai Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College. His research interest focuses on surgical and interventional treatment of structural heart disease.

Zefu Li is a Ph.D. candidate at the Chinese Academy of Medical Sciences & Peking Union Medical College. He majors in cardiovascular surgery and his project involves developing novel polymer biodegradable materials for treating structural heart disease.

Shouzheng Wang is a cardiac surgeon at the Department of Structural Heart Disease, Fuwai Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College. His research interest focuses on surgical and interventional treatment of structural heart disease.

Zhiling Luo is a chief physician at the Department of Echocardiography, Fuwai Yunnan Cardiovascular Hospital. Her current research focuses on echocardiography for congenital heart diseases and valvular diseases.

Xiangbin Pan is the director of the Department of Structural Heart Disease, Fuwai Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, and the director of the Chinese National Medical Quality Control Center for Structural Heart Disease. His research interest includes novel transcatheter therapy and device for structural heart disease.
- End -
关注我们
专业的心血管医生学术交流平台


版权及免责声明:
本网站所发表内容知识产权归属医谱平台、主办方以及原作者等相关权利人,未经许可,禁止进行复制、传播、展示、镜像、转载、摘编等。经授权使用,须注明来源,否则将追究其法律责任。有关作品内容、版权和其他问题请与本网联系。


发表留言
暂无留言
输入您的留言参与专家互动