【1】Watanabe, Hirotoshi, et al. "Effect of 1-month dual antiplatelet therapy followed by clopidogrel vs 12-month dual antiplatelet therapy on cardiovascular and bleeding events in patients receiving PCI: the STOPDAPT-2 randomized clinical trial." Jama 321.24 (2019): 2414-2427.
【2】Natsuaki, Masahiro, et al. "An aspirin-free versus dual antiplatelet strategy for coronary stenting: STOPDAPT-3 randomized trial." Circulation 149.8 (2024): 585-600.
【3】Valgimigli, Marco, Davide Cao, and Roxana Mehran. "SAFETY AND EFFICACY OF 1-MONTH VERSUS 3-MONTH DUAL ANTIPLATELET THERAPY IN HIGH BLEEDING RISK PATIENTS UNDERGOING PERCUTANEOUS CORONARY INTERVENTIONS WITH AN EVEROLIMUS ELUTING STENT: INSIGHTS FROM THE XIENCE SHORT DAPT PROGRAM." Journal of the American College of Cardiology 77.18_Supplement_1 (2021): 897-897.
【4】Krone, Ronald J., et al. "Evaluation of the American College of Cardiology/American Heart Association and the Society for Coronary Angiography and Interventions lesion classification system in the current “stent era” of coronary interventions (from the ACC-National Cardiovascular Data Registry)." The American journal of cardiology 92.4 (2003): 389-394.
【5】Capodanno, D., et al. J Am Coll Cardiol.2020;76:1468-1483.
【6】Smith Jr, Sidney C., et al. "Prevention conference VI: diabetes and cardiovascular disease: writing group VI: revascularization in diabetic patients." Circulation 105.18 (2002): e165-e169.
【7】Saito, Shigeru, et al. "BIOFLOW-IV, a randomised, intercontinental, multicentre study to assess the safety and effectiveness of the Orsiro sirolimus-eluting stent in the treatment of subjects with de novo coronary artery lesions: primary outcome target vessel failure at 12 months." EuroIntervention 15.11 (2019): e1006-e1013.
【8】Serruys, Patrick W., et al. "Comparison of zotarolimus-eluting and everolimus-eluting coronary stents." New England journal of medicine 363.2 (2010): 136-146.
【9】Zanchin, Christian, et al. "Everolimus-eluting biodegradable polymer versus everolimus-eluting durable polymer stent for coronary revascularization in routine clinical practice." JACC: Cardiovascular Interventions 12.17 (2019): 1665-1675.
【10】Shiomi, H., et al. J Am Coll Cardiol Interv.2019;12:637-647.
【11】Kufner, Sebastian, et al. "Ten-year clinical outcomes from a trial of three limus-eluting stents with different polymer coatings in patients with coronary artery disease: results from the ISAR-TEST 4 randomized trial." Circulation 139.3 (2019): 325-333.
【12】Jinnouchi, H., et al. J Am Coll Cardiol.2019;74:Suppl B – TCT-291.
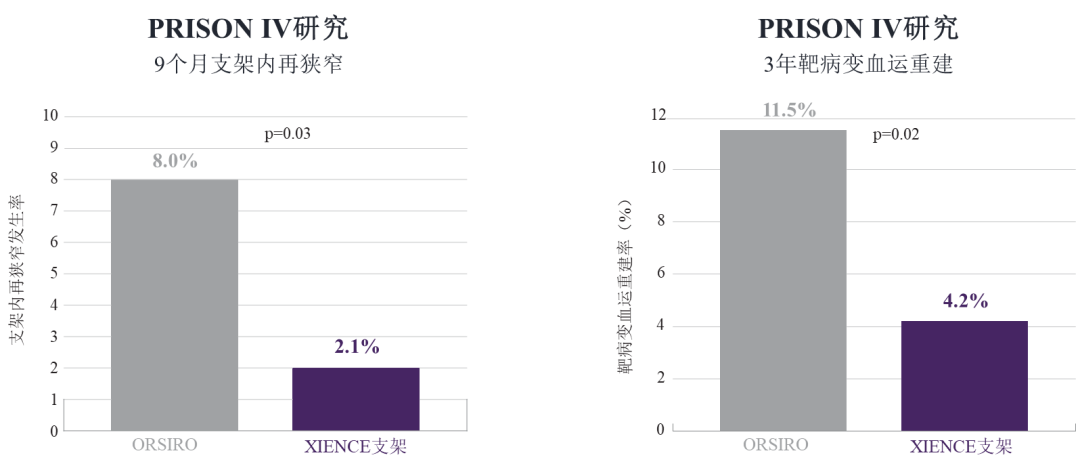
【13】Zivelonghi, Carlo, et al. "Impact of ultra‐thin struts on restenosis after chronic total occlusion recanalization: insights from the randomized PRISON IV trial." Journal of Interventional Cardiology 31.5 (2018): 580-587.
【14】Morino, Yoshihiro, et al. "Early vascular responses to everolimus-eluting cobalt–chromium stent in the culprit lesions of st-elevation myocardial infarction: results from a multicenter prospective optical coherence tomography study (MECHANISM-AMI 2-week follow-up study)." Cardiovascular intervention and therapeutics 34 (2019): 14-24.
【15】Sabate, Manel, et al. "Everolimus-eluting stent versus bare-metal stent in ST-segment elevation myocardial infarction (EXAMINATION): 1 year results of a randomised controlled trial." The Lancet 380.9852 (2012): 1482-1490.
【16】Dzivak, V., Catheter Cardiovasc Interv.2013;82(3):E163-E172.
【17】Onuma, Yoshinobu, et al. "Efficacy of everolimus eluting stent implantation in patients with calcified coronary culprit lesions: two‐year angiographic and three‐year clinical results from the SPIRIT II study." Catheterization and Cardiovascular Interventions 76.5 (2010): 634-642.
【18】Hong, S., et al. Am Coll Cardiol Intv 2016;9:1438–1446.
【19】Stone, G., at al. N Engl J Med.2016;1:1-13.
【20】Yuji Oikawa, Kenya Nasu.Presentation “CALC-ACCESS registry”, CVIT 2019.
【21】Sato, Takao, et al. "Comparison of clinical outcomes of coronary artery stent implantation in patients with end‐stage chronic kidney disease including hemodialysis for three everolimus eluting (EES) stent designs: Bioresorbable polymer‐EES, platinum chromium‐EES, and cobalt chrome‐EES." Journal of Interventional Cardiology 31.2 (2018): 170-176.
【22】Kyono, Hiroyuki, et al. "Angiographic and clinical outcomes of 100 consecutive severe calcified lesions requiring rotational atherectomy prior to sirolimus-eluting stent implantation in hemodialysis and non-hemodialysis patients." Cardiovascular intervention and therapeutics 26 (2011): 98-103.
【23】Chang, C.C., Onuma, Y., Lesiak, M., Merkulov, E., Anderson, R., Kretov, E., Barragan, P., Oldroyd, K.G. and van Geuns, R.J., 2020. Optical Coherence Tomography Assessment for Percutaneous Coronary Intervention of the Left Main Artery: The IDEAL-LM Trial. Cardiovascular Interventions, 13(3), pp.401-402.
【24】Baron, Suzanne J., et al. "Quality-of-life after everolimus-eluting stents or bypass surgery for left-main disease: results from the EXCEL trial." Journal of the American College of Cardiology 70.25 (2017): 3113-3122.




发表留言
暂无留言
输入您的留言参与专家互动